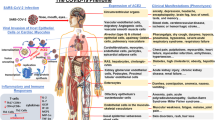
Abstract
Globally, 265,713,467 confirmed cases of SARS-CoV-2 (CoV-2), including 5,260,888 deaths, have been reported by the WHO. It is important to study the mechanism of this infectious disease. A variety of evidences show the potential association between CoV-2 and glucose metabolism. Notably, people with type 2 diabetes mellitus (T2DM) and other metabolic complications were prone to have a higher risk of developing a more severe infection course than people who were metabolically normal. The correlations between glucose metabolism and CoV-2 progression have been widely revealed. This review will discuss the association between glucose metabolism disorders and CoV-2 progression, showing the promoting effect of diabetes and other diseases related to glucose metabolism disorders on the progression of CoV-2. We will further conclude the effects of key proteins and pathways in glucose metabolism regulation on CoV-2 progression and potential interventions by targeting glucose metabolism disorders for CoV-2 treatment. Therefore, this review will provide systematic insight into the treatment of CoV-2 from the perspective of glucose metabolism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated or analyzed in this study are available from the corresponding author on reasonable request.
References
Ozkan Oktay E, Tuncay S, Kaman T, Karasakal OF, Ozcan OO, Soylamis T, et al. An update comprehensive review on the status of COVID-19: vaccines, drugs, variants and neurological symptoms. Turk J Biol. 2021;454:342–57. https://doi.org/10.3906/biy-2106-23.
Shen Q, Li J, Zhang Z, Guo S, Wang Q, An X, et al. COVID-19: systemic pathology and its implications for therapy. Int J Biol Sci. 2022;181:386–408. https://doi.org/10.7150/ijbs.65911.
Touyz RM, Boyd MOE, Guzik T, Padmanabhan S, McCallum L, Delles C, et al. Cardiovascular and renal risk factors and complications associated with COVID-19. CJC Open. 2021;310:1257–72. https://doi.org/10.1016/j.cjco.2021.05.020.
Ayres JS. A metabolic handbook for the COVID-19 pandemic. Nat Metab. 2020;27:572–85. https://doi.org/10.1038/s42255-020-0237-2.
Juanola O, Martinez-Lopez S, Frances R, Gomez-Hurtado I. Non-alcoholic fatty liver disease: metabolic, genetic, epigenetic and environmental risk factors. Int J Environ Res Public Health. 2021;18:1810. https://doi.org/10.3390/ijerph18105227.
Ealey KN, Phillips J, Sung HK. COVID-19 and obesity: fighting two pandemics with intermittent fasting. Trends Endocrinol Metab. 2021;329:706–20. https://doi.org/10.1016/j.tem.2021.06.004.
Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16 Suppl 1:S27–36. https://doi.org/10.4103/2230-8210.94253.
Jiang Y, Rubin L, Peng T, Liu L, Xing X, Lazarovici P, et al. Cytokine storm in COVID-19: from viral infection to immune responses, diagnosis and therapy. Int J Biol Sci. 2022;182:459–72. https://doi.org/10.7150/ijbs.59272.
Moolamalla STR, Balasubramanian R, Chauhan R, Priyakumar UD, Vinod PK. Host metabolic reprogramming in response to SARS-CoV-2 infection: a systems biology approach. Microb Pathog. 2021;158:105114. https://doi.org/10.1016/j.micpath.2021.105114.
Li Z, Sun C, Qin Z. Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics. 2021;1117:8322–36. https://doi.org/10.7150/thno.62378.
Morris NL, Michael DN, Crotty KM, Chang SS, Yeligar SM. Alcohol-induced glycolytic shift in alveolar macrophages is mediated by hypoxia-inducible factor-1 alpha. Front Immunol. 2022;13:865492. https://doi.org/10.3389/fimmu.2022.865492.
Xu H, He Y, Ma J, Zhao Y, Liu Y, Sun L, et al. Inhibition of pyruvate dehydrogenase kinase1 by dicoumarol enhances the sensitivity of hepatocellular carcinoma cells to oxaliplatin via metabolic reprogramming. Int J Oncol. 2020;573:733–42. https://doi.org/10.3892/ijo.2020.5098.
Thaker SK, Chapa T, Garcia G Jr., Gong D, Schmid EW, Arumugaswami V, et al. Differential metabolic reprogramming by Zika virus promotes cell death in human versus mosquito cells. Cell Metab. 2019;295:1206–16 e4. https://doi.org/10.1016/j.cmet.2019.01.024.
Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19 - a systematic review. Life Sci. 2020;254:117788. https://doi.org/10.1016/j.lfs.2020.117788.
Mullen PJ, Garcia G Jr, Purkayastha A, Matulionis N, Schmid EW, Momcilovic M, et al. SARS-CoV-2 infection rewires host cell metabolism and is potentially susceptible to mTORC1 inhibition. Nat Commun. 2021;121:1876. https://doi.org/10.1038/s41467-021-22166-4.
Bharadwaj S, Singh M, Kirtipal N, Kang SG. SARS-CoV-2 and glutamine: SARS-CoV-2 triggered pathogenesis via metabolic reprograming of glutamine in host cells. Front Mol Biosci. 2020;7:627842. https://doi.org/10.3389/fmolb.2020.627842.
Krishnan S, Nordqvist H, Ambikan AT, Gupta S, Sperk M, Svensson-Akusjarvi S, et al. Metabolic perturbation associated with COVID-19 disease severity and SARS-CoV-2 replication. Mol Cell Proteom. 2021;20:100159. https://doi.org/10.1016/j.mcpro.2021.100159.
Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;2513:228–48. https://doi.org/10.1002/path.5471.
Shang C, Liu Z, Zhu Y, Lu J, Ge C, Zhang C, et al. SARS-CoV-2 causes mitochondrial dysfunction and mitophagy impairment. Front Microbiol. 2021;12:780768. https://doi.org/10.3389/fmicb.2021.780768.
Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;175:259–60. https://doi.org/10.1038/s41569-020-0360-5.
Santos AF, Povoa P, Paixao P, Mendonca A, Taborda-Barata L. Changes in glycolytic pathway in SARS-COV 2 infection and their importance in understanding the severity of COVID-19. Front Chem. 2021;9:685196. https://doi.org/10.3389/fchem.2021.685196.
Ryu G, Shin HW. SARS-CoV-2 infection of airway epithelial cells. Immune Netw. 2021;211:e3. https://doi.org/10.4110/in.2021.21.e3.
Ardestani A, Azizi Z. Targeting glucose metabolism for treatment of COVID-19. Signal Transduct Target Ther. 2021;61:112. https://doi.org/10.1038/s41392-021-00532-4.
Lian Q, Zhang K, Zhang Z, Duan F, Guo L, Luo W, et al. Differential effects of macrophage subtypes on SARS-CoV-2 infection in a human pluripotent stem cell-derived model. Nat Commun. 2022;131:2028. https://doi.org/10.1038/s41467-022-29731-5.
Icard P, Lincet H, Wu Z, Coquerel A, Forgez P, Alifano M, et al. The key role of Warburg effect in SARS-CoV-2 replication and associated inflammatory response. Biochimie. 2021;180:169–77. https://doi.org/10.1016/j.biochi.2020.11.010.
Codo AC, Davanzo GG, Monteiro LB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1alpha/glycolysis-dependent axis. Cell Metab. 2020;323:437–46 e5. https://doi.org/10.1016/j.cmet.2020.07.007.
Vakifahmetoglu-Norberg H, Ouchida AT, Norberg E. The role of mitochondria in metabolism and cell death. Biochem Biophys Res Commun. 2017;4823:426–31. https://doi.org/10.1016/j.bbrc.2016.11.088.
Tarafdar A, Pula G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int J Mol Sci. 2018;19:1912. https://doi.org/10.3390/ijms19123824.
Rajput S, Paliwal D, Naithani M, Kothari A, Meena K, Rana S. COVID-19 and gut microbiota: a potential connection. Indian J Clin Biochem. 2021;36:1–12. https://doi.org/10.1007/s12291-020-00948-9.
Ricordi C, Pacifici F, Lanzoni G, Palamara AT, Garaci E, Della-Morte D. Dietary and protective factors to halt or mitigate progression of autoimmunity, COVID-19 and its associated metabolic diseases. Int J Mol Sci. 2021;22:226. https://doi.org/10.3390/ijms22063134.
Meidaninikjeh S, Sabouni N, Marzouni HZ, Bengar S, Khalili A, Jafari R. Monocytes and macrophages in COVID-19: friends and foes. Life Sci. 2021;269:119010. https://doi.org/10.1016/j.lfs.2020.119010.
Corrao S, Pinelli K, Vacca M, Raspanti M, Argano C. Type 2 diabetes mellitus and COVID-19: a narrative review. Front Endocrinol. 2021;12:609470. 10.3389/fendo.2021.609470.
Li G, Chen Z, Lv Z, Li H, Chang D, Lu J. Diabetes mellitus and COVID-19: associations and possible mechanisms. Int J Endocrinol. 2021;2021:7394378. https://doi.org/10.1155/2021/7394378.
Steenblock C, Schwarz PEH, Ludwig B, Linkermann A, Zimmet P, Kulebyakin K, et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;911:786–98. https://doi.org/10.1016/S2213-8587(21)00244-8.
Codo AC, Davanzo GG, Monteiro LB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1alpha/glycolysis-dependent axis. Cell Metab. 2020;323:498–9. https://doi.org/10.1016/j.cmet.2020.07.015.
Muller JA, Gross R, Conzelmann C, Kruger J, Merle U, Steinhart J, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;32:149–65. https://doi.org/10.1038/s42255-021-00347-1.
Pamukcu B. Inflammation and thrombosis in patients with COVID-19: a prothrombotic and inflammatory disease caused by SARS coronavirus-2. Anatol J Cardiol. 2020;244:224–34. https://doi.org/10.14744/AnatolJCardiol.2020.56727.
Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;171:11–30. https://doi.org/10.1038/s41574-020-00435-4.
Hoshiyama M, Li B, Yao J, Harada T, Morioka T, Oite T. Effect of high glucose on nitric oxide production and endothelial nitric oxide synthase protein expression in human glomerular endothelial cells. Nephron Exp Nephrol. 2003;952:e62–8. https://doi.org/10.1159/000073673.
Lopez L, Sang PC, Tian Y, Sang Y. Dysregulated interferon response underlying severe COVID-19. Viruses. 2020;12:1212. https://doi.org/10.3390/v12121433.
Calabretta E, Moraleda JM, Iacobelli M, Jara R, Vlodavsky I, O'Gorman P, et al. COVID-19-induced endotheliitis: emerging evidence and possible therapeutic strategies. Br J Haematol. 2021;1931:43–51. https://doi.org/10.1111/bjh.17240.
Petek BJ, Moulson N, Baggish AL, Kliethermes SA, Patel MR, Churchill TW. et al. Prevalence and clinical implications of persistent or exertional cardiopulmonary symptoms following SARS-CoV-2 infection in 3597 collegiate athletes: a study from the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA). Br J Sports Med. 2021;56:913–8. 10.1136/bjsports-2021-104644.
Rais N, Ahmad R, Ved A, Parveen K, Ishrat T, Prakash O, et al. Diabetes mellitus during the pandemic COVID-19: prevelance, pathophysiology, mechanism, and management: an updated overview. Curr Diabetes Rev. 2021;18:e120721194712. https://doi.org/10.2174/1573399817666210712160651.
Coate KC. GP73 links SARS-CoV-2 infection with dysglycaemia. Nat Metab. 2022;4:9–10. https://doi.org/10.1038/s42255-021-00511-7.
Jose A, Singh S, Roychoudhury A, Kholakiya Y, Arya S, Roychoudhury S. Current understanding in the pathophysiology of SARS-CoV-2-associated rhino-orbito-cerebral mucormycosis: a comprehensive review. J Maxillofac Oral Surg. 2021;20:1–8. https://doi.org/10.1007/s12663-021-01604-2.
Chen X, Wang Y, Tao J, Shi Y, Gai X, Huang F, et al. mTORC1 up-regulates GP73 to promote proliferation and migration of hepatocellular carcinoma cells and growth of xenograft tumors in mice. Gastroenterology. 2015;1493:741–52 e14. https://doi.org/10.1053/j.gastro.2015.05.005.
Liu Y, Zou Z, Zhu B, Hu Z, Zeng P. CXCL10 decreases GP73 expression in hepatoma cells at the early stage of hepatitis C virus (HCV) infection. Int J Mol Sci. 2013;1412:24230–41. https://doi.org/10.3390/ijms141224230.
Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;2111:e13128. https://doi.org/10.1111/obr.13128.
Vallis M, Glazer S. Protecting individuals living with overweight and obesity. Attitudes and concerns toward COVID-19 vaccination in Canada. Obesity. 2021;29:1128–1137. https://doi.org/10.1002/oby.23182.
Zhu Q, Zhang Y, Kang J, Chen Z, Peng M, Chen M, et al. Weakened humoral and cellular immune response to the inactivated COVID-19 vaccines in Chinese individuals with obesity/overweight. Genes Dis. 2023;102:608–17. https://doi.org/10.1016/j.gendis.2022.10.023.
Glazer SA, Vallis M. Weight gain, weight management and medical care for individuals living with overweight and obesity during the COVID-19 pandemic (EPOCH study). Obes Sci Pract. 2022;85:556–68. https://doi.org/10.1002/osp4.591.
Altmann DM, Boyton RJ. SARS-CoV-2 T cell immunity: specificity, function, durability, and role in protection. Sci Immunol. 2020;5:549. https://doi.org/10.1126/sciimmunol.abd6160.
Lim S, Shin SM, Nam GE, Jung CH, Koo BK. Proper management of people with obesity during the COVID-19 pandemic. J Obes Metab Syndr. 2020;292:84–98. https://doi.org/10.7570/jomes20056.
Boutin S, Hildebrand D, Boulant S, Kreuter M, Ruter J, Pallerla SR, et al. Host factors facilitating SARS-CoV-2 virus infection and replication in the lungs. Cell Mol Life Sci. 2021;7816:5953–76. https://doi.org/10.1007/s00018-021-03889-5.
Mohamed Khosroshahi L, Rezaei N. Dysregulation of the immune response in coronavirus disease 2019. Cell Biol Int. 2021;454:702–7. https://doi.org/10.1002/cbin.11517.
Krapic M, Kavazovic I, Wensveen FM. Immunological mechanisms of sickness behavior in viral infection. Viruses. 2021;13:1311. https://doi.org/10.3390/v13112245.
Piatkiewicz P, Milek T, Bernat-Karpinska M, Ohams M, Czech A, Ciostek P. The dysfunction of NK cells in patients with type 2 diabetes and colon cancer. Arch Immunol Ther Exp. 2013;613:245–53. https://doi.org/10.1007/s00005-013-0222-5.
Berrou J, Fougeray S, Venot M, Chardiny V, Gautier JF, Dulphy N, et al. Natural killer cell function, an important target for infection and tumor protection, is impaired in type 2 diabetes. PLoS ONE. 2013;84:e62418. https://doi.org/10.1371/journal.pone.0062418.
Bojkova D, Costa R, Reus P, Bechtel M, Jaboreck MC, Olmer R, et al. Targeting the pentose phosphate pathway for SARS-CoV-2 therapy. Metabolites. 2021;11:1110. https://doi.org/10.3390/metabo11100699.
Young MJ, Clyne CD, Chapman KE. Endocrine aspects of ACE2 regulation: RAAS, steroid hormones and SARS-CoV-2. J Endocrinol. 2020;2472:R45–R62. https://doi.org/10.1530/JOE-20-0260.
Memon B, Abdelalim EM. ACE2 function in the pancreatic islet: implications for relationship between SARS-CoV-2 and diabetes. Acta Physiol. 2021;2334:e13733. https://doi.org/10.1111/apha.13733.
Montefusco L, Ben Nasr M, D'Addio F, Loretelli C, Rossi A, Pastore I, et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat Metab. 2021;36:774–85. https://doi.org/10.1038/s42255-021-00407-6.
Lin CY, Wu CH, Hsu CY, Chen TH, Lin MS, Lin YS. et al. Reduced mortality associated with the use of metformin among patients with autoimmune diseases. Front Endocrinol. 2021;12:641635. 10.3389/fendo.2021.641635.
Shang C, Zhuang X, Zhang H, Li Y, Zhu Y, Lu J, et al. Inhibition of autophagy suppresses SARS-CoV-2 replication and ameliorates pneumonia in hACE2 transgenic mice and xenografted human lung tissues. J Virol. 2021;9524:e0153721. https://doi.org/10.1128/JVI.01537-21.
Zhou YW, Xie Y, Tang LS, Pu D, Zhu YJ, Liu JY, et al. Therapeutic targets and interventional strategies in COVID-19: mechanisms and clinical studies. Signal Transduct Target Ther. 2021;61:317. https://doi.org/10.1038/s41392-021-00733-x.
Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, et al. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am J Respir Crit Care Med. 2008;1782:168–79. https://doi.org/10.1164/rccm.200710-1602OC.
Silvagno F, Vernone A, Pescarmona GP. The role of glutathione in protecting against the severe inflammatory response triggered by COVID-19. Antioxidants. 2020;9:97. https://doi.org/10.3390/antiox9070624.
Xiao N, Nie M, Pang H, Wang B, Hu J, Meng X, et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat Commun. 2021;121:1618. https://doi.org/10.1038/s41467-021-21907-9.
Wang F, Zhang J, Zhou G. 2-Deoxy-D-glucose impedes T cell-induced apoptosis of keratinocytes in oral lichen planus. J Cell Mol Med. 2021;2521:10257–67. https://doi.org/10.1111/jcmm.16964.
Marin R, Pujol FH, Rojas D, Sobrevia L. SARS- CoV-2 infection and oxidative stress in early-onset preeclampsia. Biochim Biophys Acta Mol Basis Dis. 2021;18683:166321. https://doi.org/10.1016/j.bbadis.2021.166321.
Hoang BX, Shaw G, Fang W, Han B. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J Glob Antimicrob Resist. 2020;23:256–62. https://doi.org/10.1016/j.jgar.2020.09.025.
Shi Z, Puyo CA. N-Acetylcysteine to combat COVID-19: an evidence review. Ther Clin Risk Manag. 2020;16:1047–55. https://doi.org/10.2147/TCRM.S273700.
Patocka J, Kuca K, Oleksak P, Nepovimova E, Valis M, Novotny M, et al. Rapamycin: drug repurposing in SARS-CoV-2 infection. Pharmaceuticals. 2021;14:143. https://doi.org/10.3390/ph14030217.
Shi G, Chiramel AI, Li T, Lai KK, Kenney AD, Zani A, et al. Rapalogs downmodulate intrinsic immunity and promote cell entry of SARS-CoV-2. The J. Clin. Investig. 2022;132:e160766. https://doi.org/10.1172/JCI160766.
Hoepel W, Chen HJ, Geyer CE, Allahverdiyeva S, Manz XD, de Taeye SW, et al. High titers and low fucosylation of early human anti-SARS-CoV-2 IgG promote inflammation by alveolar macrophages. Sci Transl Med. 2021;13:13596. https://doi.org/10.1126/scitranslmed.abf8654.
Gilbert MP, Pratley RE. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front Endocrinol. 2020;11:178. https://doi.org/10.3389/fendo.2020.00178.
Goker H, Aladag Karakulak E, Demiroglu H, Ayaz Ceylan CM, Buyukasik Y, Inkaya AC, et al. The effects of blood group types on the risk of COVID-19 infection and its clinical outcome. Turk J Med Sci. 2020;504:679–83. https://doi.org/10.3906/sag-2005-395.
Chitadze G, Wehkamp U, Janssen O, Bruggemann M, Lettau M. The serine protease CD26/DPP4 in non-transformed and malignant T cells. Cancers. 2021;13:1323. https://doi.org/10.3390/cancers13235947.
Rohrborn D, Wronkowitz N, Eckel J. DPP4 in diabetes. Front Immunol. 2015;6:386. https://doi.org/10.3389/fimmu.2015.00386.
Xu C, Wang A, Marin M, Honnen W, Ramasamy S, Porter E, et al. Human defensins inhibit SARS-CoV-2 infection by blocking viral entry. Viruses. 2021;13:137. https://doi.org/10.3390/v13071246.
Zhou L, Ivanov R II, Spolski R, Min K, Shenderov T. Egawa, et al. IL-6 programs T(H)−17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;89:967–74. https://doi.org/10.1038/ni1488.
Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;151:43–58. https://doi.org/10.1096/fj.99-1003rev.
Ma X, Liu Z, Ilyas I, Little PJ, Kamato D, Sahebka A, et al. GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int J Biol Sci. 2021;178:2050–68. https://doi.org/10.7150/ijbs.59965.
Brunton S. GLP-1 receptor agonists vs. DPP-4 inhibitors for type 2 diabetes: is one approach more successful or preferable than the other? Int J Clin Pract. 2014;685:557–67. https://doi.org/10.1111/ijcp.12361.
Kawasaki T, Chen W, Htwe YM, Tatsumi K, Dudek SM. DPP4 inhibition by sitagliptin attenuates LPS-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2018;3155:L834–L845. https://doi.org/10.1152/ajplung.00031.2018.
Scheen AJ. DPP-4 inhibition and COVID-19: from initial concerns to recent expectations. Diabetes Metab. 2021;472:101213. https://doi.org/10.1016/j.diabet.2020.11.005.
Dalan R, Ang LW, Tan WYT, Fong SW, Tay WC, Chan YH, et al. The association of hypertension and diabetes pharmacotherapy with COVID-19 severity and immune signatures: an observational study. Eur Heart J Cardiovasc Pharmacother. 2021;73:e48–e51. https://doi.org/10.1093/ehjcvp/pvaa098.
Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;641:24–34. https://doi.org/10.1016/j.metabol.2014.08.004.
Reidy SP, Weber J. Leptin: an essential regulator of lipid metabolism. Comp Biochem Physiol A Mol Integr Physiol. 2000;1253:285–98. https://doi.org/10.1016/s1095-6433(00)00159-8.
Nouri-Keshtkar M, Taghizadeh S, Farhadi A, Ezaddoustdar A, Vesali S, Hosseini R, et al. Potential impact of diabetes and obesity on alveolar type 2 (AT2)-lipofibroblast (LIF) interactions after COVID-19 infection. Front Cell Dev Biol. 2021;9:676150. https://doi.org/10.3389/fcell.2021.676150.
Wang J, Xu Y, Zhang X, Wang S, Peng Z, Guo J, et al. Leptin correlates with monocytes activation and severe condition in COVID-19 patients. J Leukoc Biol. 2021;1101:9–20. https://doi.org/10.1002/JLB.5HI1020-704R.
Hoffmann M, Hofmann-Winkler H, Smith JC, Krüger N, Arora P, Sørensen LK, et al. Camostat mesylate inhibits SARSCoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine. 2021;65:103255. https://doi.org/10.1016/j.ebiom.2021.103255.
Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database Syst Rev. 2021;2:CD013587. https://doi.org/10.1002/14651858.CD013587.pub2.
Wondafrash DZ, Desalegn TZ, Yimer EM, Tsige AG, Adamu BA, Zewdie KA. Potential effect of hydroxychloroquine in diabetes mellitus: a systematic review on preclinical and clinical trial studies. J Diabetes Res. 2020;2020:5214751. https://doi.org/10.1155/2020/5214751.
Mercado-Gomez M, Prieto-Fernandez E, Goikoetxea-Usandizaga N, Vila-Vecilla L, Azkargorta M, Bravo M, et al. The spike of SARS-CoV-2 promotes metabolic rewiring in hepatocytes. Commun Biol. 2022;51:827. https://doi.org/10.1038/s42003-022-03789-9.
Kalra S, Kalra B, Agrawal N, Unnikrishnan A. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr. 2011;31:2. https://doi.org/10.1186/1758-5996-3-2.
Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;427:1048–59. https://doi.org/10.1345/aph.1K615.
Yin W, Mao C, Luan X, Shen DD, Shen Q, Su H, et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;3686498:1499–504. https://doi.org/10.1126/science.abc1560.
Li YN, Su Y. Remdesivir attenuates high fat diet (HFD)-induced NAFLD by regulating hepatocyte dyslipidemia and inflammation via the suppression of STING. Biochem Biophys Res Commun. 2020;5262:381–8. https://doi.org/10.1016/j.bbrc.2020.03.034.
Gubernatorova EO, Gorshkova EA, Polinova AI, Drutskaya MS. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020;53:13–24. https://doi.org/10.1016/j.cytogfr.2020.05.009.
Roskoski R Jr. Janus kinase (JAK) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol Res. 2016;111:784–803. https://doi.org/10.1016/j.phrs.2016.07.038.
Egbuonu F, Antonio FA, Edavalath M. Effect of inhaled corticosteroids on glycemic status. Open Respir Med J. 2014;8:101–5. https://doi.org/10.2174/1874306401408010101.
Crisan Dabija R, Antohe I, Trofor A, Antoniu SA. Corticosteroids in SARS-COV2 infection: certainties and uncertainties in clinical practice. Expert Rev Anti Infect Ther. 2021;1912:1553–62. https://doi.org/10.1080/14787210.2021.1933437.
Campione E, Lanna C, Cosio T, Rosa L, Conte MP, Iacovelli F, et al. Lactoferrin against SARS-CoV-2: in vitro and in silico evidences. Front Pharmacol. 2021;12:666600. https://doi.org/10.3389/fphar.2021.666600.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82204389 & 31900502), the Natural Science Foundation of Henan (No. 222300420284), the Henan Medical Science and Technology Joint Building Program (No. LHGJ20200310 & No. LHGJ20190236 & No. LHGJ20190227).
Author information
Authors and Affiliations
Contributions
Yi Luan, Ying Luan, KDR and YY conceptualized and wrote the manuscript and created Figures. YY, and KDR contributed to the writing of the manuscript. Yi Luan, Ying Luan, HL, HH, BJ, BQ, and KDR reviewed and modified the manuscript. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luan, Y., Luan, Y., He, H. et al. Glucose metabolism disorder: a potential accomplice of SARS-CoV-2. Int J Obes 47, 893–902 (2023). https://doi.org/10.1038/s41366-023-01352-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01352-y