Abstract
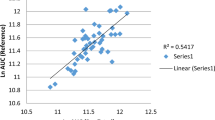
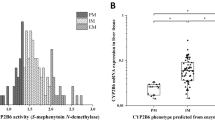
Direct-acting antivirals, asunaprevir (ASV), daclatasvir (DCV), and beclabuvir (BCV) are known to be mainly metabolized by CYP3A enzymes; however, the differences in the detailed metabolic activities of CYP3A4 and CYP3A5 on these drugs are not well clarified. The aim of the present study was to elucidate the relative contributions of CYP3A4 and CYP3A5 to the metabolism of ASV, DCV, and BCV, as well as the effect of CYP3A5*3 genetic variant in vitro. The amount of each drug and their major metabolites were determined using LC-MS/MS. Recombinant CYP3As and CYP3A5*3-genotyped human liver microsomes (CYP3A5 expressers or non-expressers) were used for the determination of their metabolic activities. The contribution of CYP3A5 to ASV metabolism was considerable compared to that of CYP3A4. Consistently, ASV metabolic activity in CYP3A5 expressers was higher than those in CYP3A5 non-expresser. Moreover, CYP3A5 expression level was significantly correlated with ASV metabolism. In contrast, these observations were not found in DCV and BCV metabolism. To our knowledge, this is the first study to directly demonstrate the effect of CYP3A5*3 genetic variants on the metabolism of ASV. The findings of the present study may provide basic information on ASV, DCV, and BCV metabolisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87.
Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–57.
Bartenschlager R, Lohmann V, Penin F. The molecular and structural basis of advanced antiviral therapy for hepatitis C virus infection. Nat Rev Microbiol. 2013;11:482–96.
Kumada H, Suzuki F, Suzuki Y, Toyota J, Karino Y, Chayama K, et al. Randomized comparison of daclatasvir+asunaprevir versus telaprevir+peginterferon/ribavirin in Japanese hepatitis C virus patients. J Gastroenterol Hepatol. 2016;31:14–22.
Pasquinelli C, McPhee F, Eley T, Villegas C, Sandy K, Sheridan P, et al. Single- and multiple-ascending-dose studies of the NS3 protease inhibitor asunaprevir in subjects with or without chronic hepatitis C. Antimicrob Agents Chemother. 2012;56:1838–44.
Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, et al. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55:742–8.
Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083–91.
McPhee F, Hernandez D, Yu F, Ueland J, Monikowski A, Carifa A, et al. Resistance analysis of hepatitis C virus genotype 1 prior treatment null responders receiving daclatasvir and asunaprevir. Hepatology. 2013;58:902–11.
McPhee F, Suzuki Y, Toyota J, Karino Y, Chayama K, Kawakami Y, et al. High sustained virologic response to daclatasvir plus asunaprevir in elderly and cirrhotic patients with hepatitis C virus genotype 1b without baseline NS5A polymorphisms. Adv Ther. 2015;32:637–49.
Lemm JA, Liu M, Gentles RG, Ding M, Voss S, Pelosi LA, et al. Preclinical characterization of BMS-791325, an allosteric inhibitor of hepatitis C Virus NS5B polymerase. Antimicrob Agents Chemother. 2014;58:3485–95.
Pelosi LA, Voss S, Liu M, Gao M, Lemm JA. Effect on hepatitis C virus replication of combinations of direct-acting antivirals, including NS5A inhibitor daclatasvir. Antimicrob Agents Chemother. 2012;56:5230–9.
McPhee F, Hernandez D, Zhou N, Ueland J, Yu F, Vellucci V. Pooled analysis of HCV genotype 1 resistance-associated substitutions in NS5A, NS3 and NS5B pre-and post-treatment with 12 weeks of daclatasvir, asunaprevir and beclabuvir. Antivir Ther. 2018;23:53–66.
Poordad F, Sievert W, Mollison L, Bennett M, Tse E, Bräu N, et al. Fixed-dose combination therapy with daclatasvir, asunaprevir, and beclabuvir for noncirrhotic patients with HCV genotype 1 infection. JAMA. 2015;313:1728–35.
Daly AK. Significance of the minor cytochrome P450 3A isoforms. Clin. Pharmacokinet. 2006;45:13–31.
Westlind A, Malmebo S, Johansson I, Otter C, Andersson TB, Ingelman-Sundberg M, et al. Cloning and tissue distribution of a novel human cytochrome p450 of the CYP3A subfamily, CYP3A43. Biochem. Biophys. Res. Commun. 2001;281:1349–55.
Williams JA, Ring BJ, Cantrell VE, Jones DR, Eckstein J, Ruterbories K, et al. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab Dispos. 2002;30:883–91.
Niwa T, Murayama N, Emoto C, Yamazaki H. Comparison of kinetic parameters for drug oxidation rates and substrate inhibition potential mediated by cytochrome P450 3A4 and 3A5. Curr Drug Metab. 2008;9:20–33.
Dai Y, Hebert MF, Isoherranen N, Davis CL, Marsh C, Shen DD, et al. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos. 2006;34:836–47.
Jacobson PA, Oetting WS, Brearley AM, Leduc R, Guan W, Schladt D, et al. Novel polymorphisms associated with tacrolimus trough concentrations: results from a multicenter kidney transplant consortium. Transplantation. 2011;91:300–8.
Niioka T, Kagaya H, Saito M, Inoue T, Numakura K, Habuchi T, et al. Capability of utilizing CYP3A5 polymorphisms to predict therapeutic dosage of tacrolimus at early stage post-renal transplantation. Int. J. Mol. Sci. 2015;16:1840–54.
Hsu MH, Savas U, Johnson EF. The X-Ray crystal structure of the human mono-oxygenase cytochrome P450 3A5-ritonavir complex reveals active site differences between P450s 3A4 and 3A5. Mol. Pharm. 2018;93:14–24.
Gong J, Eley T, He B, Arora V, Philip T, Jiang H, et al. Characterization of ADME properties of [14C] asunaprevir (BMS-650032) in humans. Xenobiotica. 2016;46:52–64.
Li W, Zhao W, Liu X, Huang X, Lopez OD, Leet JE, et al. Biotransformation of daclatasvir in vitro and in nonclinical species: formation of the main metabolite by pyrrolidine δ-oxidation and rearrangement. Drug Metab Dispos. 2016;44:809–20.
Garimella T, Tao X, Sims K, Chang YT, Rana J, Myers E, et al. Effects of a fixed-dose co-formulation of daclatasvir, asunaprevir, and beclabuvir on the pharmacokinetics of a cocktail of cytochrome P450 and drug transporter substrates in healthy subjects. Drugs R D. 2018;18:55–65.
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–91.
Toyota J, Karino Y, Suzuki F, Ikeda F, Ido A, Tanaka K, et al. Daclatasvir/asunaprevir/beclabuvir fixed-dose combination in Japanese patients with HCV genotype 1 infection. J Gastroenterol. 2017;52:385–95.
San SN, Matsumoto J, Saito Y, Koike M, Sakaue H, Kato Y, et al. Minor contribution of CYP3A5 to the metabolism of hepatitis C protease inhibitor paritaprevir in vitro. Xenobiotica. 2019;49:935–44.
Walsky RL, Obach RS, Hyland R, Kang P, Zhou S, West M, et al. Selective mechanism-based inactivation of CYP3A4 by CYP3cide (PF-04981517) and its utility as an in vitro tool for delineating the relative roles of CYP3A4 versus CYP3A5 in the metabolism of drugs. Drug Metab Dispos. 2012;40:686–97.
Yuan L, Jiang H, Zheng N, Xia YQ, Ouyang Z, Zeng J, et al. A validated LC-MS/MS method for the simultaneous determination of BMS-791325, a hepatitis C virus NS5B RNA polymerase inhibitor, and its metabolite in plasma. J Chromatogr B Anal Technol Biomed Life Sci. 2014;15(973C):1–8.
Houston JB, Kenworthy KE. In vitro-in vivo scaling of CYP kinetic data not consistent with the classical Michaelis-Menten model. Drug Metab Dispos. 2000;28:246–54.
Uchida Y, Naiki K, Kouyama JI, Sugawara K, Nakao M, Motoya D, et al. Serum asunaprevir concentrations showing correlation with the extent of liver fibrosis as a factor inducing liver injuries in patients with genotype-1b hepatitis C virus receiving daclatasvir plus asunaprevir therapy. PLoS ONE. 2018;13:e0205600.
Akuta N, Sezaki H, Suzuki F, Kawamura Y, Hosaka T, Kobayashi M, et al. Relationships between serum asunaprevir concentration and alanine aminotransferase elevation during daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. J Med Virol. 2016;88:506–11.
Eley T, He B, Chang I, Colston E, Child M, Bedford W, et al. The effect of hepatic impairment on the pharmacokinetics of asunaprevir, an HCV NS3 protease inhibitor. Antivir Ther. 2015;20:29–37.
Lin YS, Dowling AL, Quigley SD, Farin FM, Zhang J, Lamba J, et al. Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Mol Pharm. 2002;62:162–72.
Poole RM. Daclatasvir + asunaprevir: first global approval. Drugs. 2014;74:1559–71.
Mosure KW, Knipe JO, Browning M, Arora V, Shu YZ, Phillip T, et al. Preclinical pharmacokinetics and in vitro metabolism of asunaprevir (BMS-650032), a potent hepatitis C virus NS3 protease inhibitor. J Pharm Sci. 2015;104:2813–23.
Dogra A, Bhatt S, Magotra A, Sharma A, Kotwal P, Gour A, et al. Intervention of curcumin on oral pharmacokinetics of daclatasvir in rat: a possible risk for long-term use. Phytother Res. 2018;32:1967–74.
Harada N, Yoshizumi T, Ikegami T, Itoh S, Furusho N, Kato M, et al. Serum asunaprevir and daclatasvir concentrations and outcomes in patients with recurrent hepatitis C who have undergone living donor liver transplantation. Anticancer Res. 2018;38:5513–20.
Bifano M, Adamczyk R, Hwang C, Kandoussi H, Marion A, Bertz RJ. An open-label investigation into drug–drug interactions between multiple doses of daclatasvir and single-dose cyclosporine or tacrolimus in healthy subjects. Clin Drug Investig. 2015;35:281–9.
Furihata T, Matsumoto S, Fu Z, Tsubota A, Sun Y, Matsumoto S, et al. Different interaction profiles of direct-acting anti-hepatitis C virus agents with human organic anion transporting polypeptides. Antimicrob Agents Chemother. 2014;58:4555–64.
Eley T, Han YH, Huang SP, He B, Li W, Bedford W, et al. Organic anion transporting polypeptide-mediated transport of, and inhibition by, asunaprevir, an inhibitor of hepatitis C virus NS3 protease. Clin Pharm Ther. 2015;97:159–66.
Shitara Y, Maeda K, Ikejiri K, Yoshida K, Horie T, Sugiyama Y. Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: their roles in hepatic clearance and intestinal absorption. Biopharm Drug Dispos. 2013;34:45–78.
Nakanishi T, Tamai I. Interaction of drug or food with drug transporters in intestine and liver. Curr Drug Metab. 2015;16:753–64.
Saravanakumar A, Sadighi A, Ryu R, Akhlaghi F. Physicochemical properties, biotransformation, and transport pathways of established and newly approved medications: a systematic review of the top 200 most prescribed drugs vs. the FDA-approved drugs between 2005 and 2016. Clin Pharmacokinet. 2019;58:1281–94.
Acknowledgements
We acknowledge Drs. Yasuyuki Momose (International University of Health and Welfare, IUHW), Kayoko Maezawa (IUHW), Takeshi Ito (IUHW), and Natsuko Sugiyama (IUHW) for providing useful suggestions. We thank Editage (www.editage.jp) for English language editing. This work was supported by the JSPS KAKENHI (Grant-in-Aid for Young Scientists (B), Grant Number: 16K18955).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Matsumoto, J., San, S.N., Fujiyoshi, M. et al. Effect of CYP3A5*3 genetic variant on the metabolism of direct-acting antivirals in vitro: a different effect on asunaprevir versus daclatasvir and beclabuvir. J Hum Genet 65, 143–153 (2020). https://doi.org/10.1038/s10038-019-0685-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-019-0685-2