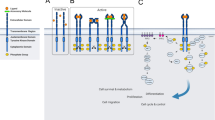
Abstract
The epidermal growth factor receptor (EGFR) is a transmembrane receptor with tyrosine kinase activity involved in regulation of cellular multiplication, survival, differentiation and metastasis. Our knowledge about function and complex management of these receptors has driving the development of specific and targeted treatment modalities for human cancers in the last 20 years. EGFR is the first receptor target against which monoclonal antibodies (mAb) have been evolved for cancer treatment. Here we review the biology of ErbB receptors, including their architecture, signaling, regulation and therapeutic strategies and the mechanisms of resistances offered by the receptors against small-molecule tyrosine kinases and resistance overcome implications of mAbs. The efficacy of EGFR-specific mAb in cancer depends on site specific extracellular region of EGFR, which has crucial role in process of dimerization and activation. This review highlights evolution of various resistance mechanisms due to consequences of current small-molecule anti-EGFR therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 2002; 110: 775–787.
Downward J, Yarden Y, Mayes E, Scrace G, Totty N, Stockwell P et al. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature 1984; 307: 521–527.
Johns TG, Stockert E, Ritter G, Jungbluth AA, Huang HJ, Cavenee WK et al. Novel monoclonal antibody specific for the de2–7 epidermal growth factor receptor (EGFR) that also recognizes the EGFR expressed in cells containing amplification of the EGFR gene. Int J Cancer 2002; 98: 398–408.
Klapper LN, Kirschbaum MH, Sela M, Yarden Y . Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv Cancer Res 2000; 77: 25–79.
Mendelsohn J . The epidermal growth factor receptor as a target for cancer therapy. Endocr Relat Cancer 2001; 8: 3–9.
Arteaga CL, Baselga J . Clinical trial design and end points for epidermal growth factor receptor-targeted therapies: implications for drug development and practice. Clin Cancer Res 2003; 9: 1579–1589.
Rubin MS, Shin DM, Pasmantier M, Falcey JW, Paulter VJ, Fetzer KM et al. The EGF receptor family as targets for cancer therapy. Proc Annu Meet Am Soc Clin Oncol 2000; 19: 474–489.
Yuriko I, Hiroyuki N . ErbB1-4-dependent EGF/neuregulin signals and their cross talk in the central nervous system: pathological implications in schizophrenia and Parkinson's disease. Front Cell Neurosci 2013; 7: 414–428.
Dancey JE, Freidlin B . Targeting epidermal growth factor receptor—are we missing the mark? Lancet 2003; 362: 62–64.
Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell 2002; 110: 763–773.
Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 2002; 110: 775–787.
Elleman TC, Domagala T, McKern NM, Nerrie M, Lonnqvist B, Adams TE et al. Identification of a determinant of epidermal growth factor receptor ligand-binding specificity using a truncated, high-affinity form of the ectodomain. Biochemistry 2001; 40: 8930–8939.
Lax I, Burgess WH, Bellot F, Ullrich A, Schlessinger J, Givol D . Localization of a major receptor-binding domain for epidermal growth factor by affinity labelling. Mol Cell Biol 1988; 8: 1831–1834.
Bellot F, Howk R, Ullrich A, Givol D, Schlessinger J . Functional analysis of the ligand binding site of EGF-receptor utilizing chimeric chicken/human receptor molecules. EMBO J 1989; 8: 421–427.
De Larco JE, Reynolds R, Carlberg K, Engle C, Todaro GJ . Sarcoma growth factor from mouse sarcoma virus-transformed cells, purification by binding and elution from epidermal growth factor receptor-rich cells. J Biol Chem 1980; 255: 3685–3690.
Yamabhai M, Anderson RG . Second cysteine-rich region of epidermal growth factor receptor contains targeting information for caveolae/rafts. J Biol Chem 2002; 277: 24843–24846.
Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 1984; 309: 418–425.
Kil SJ, Carlin C . EGF receptor residues leu(679), leu(680) mediate selective sorting of ligand-receptor complexes in early endosomal compartments. J Cell Physiol 2000; 185: 47–60.
Castagnino P, Biesova Z, Wong WT, Fazioli F, Gill GN, Di Fiore PP . Direct binding of eps8 to the juxtamembrane domain of EGFR is phosphotyrosine- and SH2-independent. Oncogene 1995; 10: 723–729.
Li H, Villalobo A . Evidence for the direct interaction between calmodulin and the human epidermal growth factor receptor. Biochem J 2002; 362: 499–505.
Lombardo CR, Consler TG, Kassel DB . In-vitro phosphorylation of the epidermal growth factor receptor autophosphorylation domain by csrc: identification of phosphorylation sites and c-src SH2 domain binding sites. Biochemistry 1995; 34: 16456–16466.
Thien CB, Langdon WY . Cbl: many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol 2001; 2: 294–307.
Athale CA, Deisboeck TS . The effects of EGF-receptor density on multiscale tumor growth patterns. J Theor Biol 2006; 238: 771–779.
El-Obeid A, Hesselager G, Westermark B, Nister M . TGF-alpha-driven tumor growth is inhibited by an EGF receptor tyrosine kinase inhibitor. Biochem Biophys Res Commun 2002; 290: 349–358.
Dawson JP, Berger MB, Lin CC, Schlessinger J, Lemmon MA, Ferguson KM . Epidermal growth factor receptor dimerization and activation require ligand-induced conformational changes in the dimer interface. Mol Cell Biol 2005; 25: 7734–7742.
Valley CC, Arndt-Jovin DJ, Karedla N, Steinkamp MP, Chizhik AI, Hlavacek WS et al. Enhanced dimerization drives ligand-independent activity of mutant epidermal growth factor receptor in lung cancer. Mol Biol Cell 2015; 26: 4087–4099.
Wieduwilt MJ, Moasser MM . The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell Mol Life Sci 2008; 65: 1566–1584.
Alexander S, Duex JE . Quantitative analysis of endocytosis and turnover of epidermal growth factor (EGF) and EGF receptor. Curr Protoc Cell Biol 2010; 67: 1236–1243.
Emrah E, Michelle MC, Mackey AM, Rameh LE, Blenis J . AKT facilitates EGFR trafficking and degradation by phosphorylating and activating PIKfyve. Sci Signal 2013; 11: 791–803.
Ferguson KM, Darling PJ, Mohan MJ, Macatee TL, Lemmon MA . Extracellular domains drive homo- but not hetero-dimerization of erbB receptors. EMBO J 2000; 19: 4632–4643.
Huang GC, Ouyang X, Epstein RJ . Proxy activation of protein ErbB2 by heterologous ligands implies a heterotetrameric mode of receptor tyrosine kinase interaction. Biochem J 1998; 331: 113–119.
Greulich H, Chen TH, Feng W, Janne PA, Alvarez JV, Zappaterra M et al. Oncogenic transformation by inhibitor-sensitive and –resistant EGFR mutants. PLoS Med 2005; 2: 301–313.
Wells A . EGF receptor. Int J Biochem Cell Biol 1999; 31: 637–643.
Nicholson RI, Gee JM, Harper ME . EGFR and cancer prognosis. Eur J Cancer 2001; 37: 9–15.
Merlino GT, Xu YH, Richert N, Clark AJ, Ishii S, Banks-Schlegel S et al. Elevated epidermal growth factor receptor gene copy number and expression in a squamous carcinoma cell line. J Clin Invest 1985; 75: 1077–1079.
Sainsbury JR, Nicholson S, Angus B, Farndon JR, Malcolm AJ, Harris AL . Epidermal growth factor receptor status of histological subtypes of breast cancer. Br J Cancer 1988; 58: 458–460.
Costa S, Stamm H, Almendral A, Ludwig H, Wyss R, Fabbro D et al. Predictive value of EGF receptor in breast cancer. Lancet 1988; 2: 1258.
Derynck R, Goeddel DV, Ullrich A, Gutterman JU, Williams RD, Bringman TS . Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res 1987; 47: 707–712.
Ibrahim SO, Vasstrand EN, Liavaag PG, Johannessen AC, Lillehaug JR . Expression of c-erbB proto-oncogene family members in squamous cell carcinoma of the head and neck. Anticancer Res 1997; 17: 4539–4546.
Aaronson SA . Growth factors and cancer. Science 1991; 254: 1146–1153.
Grunwald V, Hidalgo M . The epidermal growth factor receptor: a new target for anticancer therapy. Curr Probl Cancer 2002; 26: 109–164.
Sainsbury JR, Nicholson S, Angus B, Farndon JR, Malcolm AJ, Harris AL . Epidermal growth factor receptor status of histological subtypes of breast cancer. Br J Cancer 1988; 58: 458–460.
Brand TM, Mari I, Wheeler DL . Molecular mechanisms of resistance to the EGFR monoclonal antibody cetuximab. Cancer Biol Ther 2011; 11: 777–792.
Parthasarathy S, Moorthy P, Ponnusamy L, Dhanya H, Jain M, AparK G et al. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets 2012; 16: 15–31.
Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C . Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 1995; 378: 394–398.
Andrea L, Marschall J, Frenzel A, Schirrmann T, Schüngel M, Stefan D . Targeting antibodies to the cytoplasm. MAbs 2011; 3: 3–16.
Wheeler DL, Dunn EF, Harari PM . Understanding resistance to EGFR inhibitors—impact on future treatment strategies. Nat Rev Clin Oncol 2010; 7: 493–507.
Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R . FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist 2004; 8: 303–306.
Noemí R, Andrés F, Cardona E, Rafael R . Role of erlotinib in first-line and maintenance treatment of advanced non-small-cell lung cancer. Cancer Manag Res 2010; 2: 143–156.
Stewart EL, Tan S, Geoffrey L, Sound MT . Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations—a review. Transl Lung Cancer Res 2015; 4: 67–81.
Yun C, Mengwasser KE, Michele S, Heidi G, Kwok-Kin W et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci USA 2008; 105: 2070–2075.
Lax I, Burgess WH, Bellot F, Ullrich A, Schlessinger J, Givol D . Localization of a major receptor-binding domain for epidermal growth factor by affinity labelling. Mol Cell Bio 1988; 8: 1831–1834.
Forde PM, Ettinger DS . Managing acquired resistance in EGFR-mutated non–small cell lung cancer. Clin Adv Hematol Oncol 2015; 13: 1236–1241.
Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JM et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 2002; 110: 775–787.
Hong YS, Jang WJ, Chun KS, Jeong CH . Hsp90 inhibition by WK88-1 potently suppresses the growth of gefitinib-resistant H1975 cells harboring the T790M mutation in EGFR. Oncol Rep 2014; 31: 2619–2624.
Darren C, Susan E, Ashton S, Serban G, Cath E, Caroline A . AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014; 4: 1046–1061.
Kim SM, Kwon OJ, Hong YK, Kim JH, Solca F, Ha SJ et al. Activation of IL-6R/JAK1/STAT3 signaling induces de novo resistance to irreversible EGFR inhibitors in non-small cell lung cancer with T790M resistance mutation. Mol Cancer Ther 2012; 11: 2254–2264.
Yao Z, Fenoglio S, Gao DC, Camiolo M, Stiles B, Lindsted T et al. TGF-β IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci USA 2010; 107: 15535–15540.
Woo HS, Ahn HK, Lee HY, Park I, Kim YS, Hong J et al. Epidermal growth factor receptor (EGFR) exon 20 mutations in non-small-cell lung cancer and resistance to EGFR-tyrosine kinase inhibitors. Invest New Drugs 2014; 32: 1311–1318.
Khan NA, Mirshahidi S, Mirshahidi HR . A novel insertion mutation on exon 20 of epidermal growth factor receptor, conferring resistance to erlotinib. Case Rep Oncol 2014; 7: 491–498.
Qin B, Ariyama H, Baba E, Tanaka R, Kusaba H, Harada M et al. Activated Src and Ras induce gefitinib resistance by activation of signaling pathways downstream of epidermal growth factor receptor in human gallbladder adenocarcinoma cells. Cancer Chemother Pharmacol 2006; 58: 577–584.
Yokoyama H, Ikehara Y, Kodera Y, Ikehara S, Yatabe Y, Mochizuki Y et al. Molecular basis for sensitivity and acquired resistance to gefitinib in HER2-overexpressing human gastric cancer cell lines derived from liver metastasis. Br J Cancer 2006; 4: 1504–1513.
Ju L, Zhou C . Association of integrin beta1 and c-MET in mediating EGFR TKI gefitinib resistance in non-small cell lung cancer. Cancer Cell Int 2013; 13: 1523–1536.
Deng QF, Su BO, Zhao YM, Tang L, Zhang J, Zhou CC . Integrin β1-mediated acquired gefitinib resistance in non-small cell lung cancer cells occurs via the phosphoinositide 3-kinase-dependent pathway. Oncol Lett 2016; 1: 535–542.
Ahmed T, Patrick C . Cancer Genes in Lung Cancer Racial Disparities: Are There Any? Genes Cancer 2012; 3: 467–480.
Eser S, Schnieke A, Schneider G, Saur D . Oncogenic KRAS signalling in pancreatic cancer. British Journal of Cancer 2014; 111: 817–822.
Wang L, Hu H, Pan Y, Wang R, Li Y, Shen L et al. PIK3CA mutations frequently coexist with EGFR/KRAS mutations in non-small cell lung cancer and suggest poor prognosis in EGFR/KRAS wildtype subgroup. PLoS One 2014; 9: 88291–88298.
Suda K, Mizuuchi H, Murakami I, Uramoto H, Tanaka F, Sato K et al. CRKL amplification is rare as a mechanism for acquired resistance to kinase inhibitors in lung cancers with epidermal growth factor receptor mutation. Lung Cancer 2014; 85: 147–151.
Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 2012; 44: 852–860.
Bidkhori G, Moeini A, Masoudi-Nejad A . Modeling of tumor progression in NSCLC and intrinsic resistance to TKI in loss of PTEN expression. PLoS One 2012; 7: 48004–48011.
Sos ML, Koker M, Weir BA, Heynck S, Rabinovsky R, Zander T et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res 2009; 69: 3256–3261.
Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest 2008; 118: 2609–2619.
Peled N, Wynes MW, Ikeda N, Ohira T, Yoshida K, Qian J . Insulin like growth factor-1 receptor (IGF-1R) as a biomarker for resistance to the tyrosine kinase inhibitor gefitinib in non-small cell lung cancer. Cell Oncol 2013; 36: 277–288.
Pedersen MW, Pedersen N, Ottesen LH, Poulsen HS . Differential response to gefitinib of cells expressing normal EGFR and the mutant EGFRvIII. Br J Cancer 2005; 93: 915–923.
Nicola M, Tonia C, Maurizio M, Quintino G, Alessandris D . Expression of EGFRvIII in glioblastoma: prognostic significance revisited. Neoplasia 2011; 13: 1113–1121.
Bonavia R, Inda MM, Vandenberg S, Cheng SY, Nagane M, Hadwiger P et al. EGFRvIII promotes glioma angiogenesis and growth through the NF-κB, interleukin-8 pathway. Oncogene 2012; 31: 4054–4066.
Lihua H, Fu Liwu . Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B 2015; 5: 390–401.
Michael P, Scheid E, Michael P . Phosphoinositide-dependent phosphorylation of PDK1 regulates nuclear translocation. Mol Cell Biol 2005; 25: 2347–2363.
Wu K, Chang Q, Lu Y, Qiu P, Chen B, Thakur C et al. Gefitinib resistance resulted from STAT3-mediated Akt activation in lung cancer cells. Oncotarget 2013; 4: 2430–2438.
Nandini D, Casey W, Brain LJ, Pradip D . A critical role for HER3 in HER2-amplified and non-amplified breast cancers: function of a kinase-dead RTK. Am J Transl Res 2015; 7: 733–750.
Ultan MD, Raju VP, James GC, Nathanael SG, Jeff S . Acquired resistance to MET kinase inhibition in MET-dependent non-small cell lung cancer cells mediated by a switch to EGFR dependency. Cancer Res 2010; 15: 1625–1634.
Altomare DA, Khaled AR . Homeostasis and the importance for a balance between AKT/mTOR activity and intracellular signaling. Curr Med Chem 2012; 19: 3748–3762.
Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest 2008; 18: 2609–2619.
Sahitya KD, Olumuyiwa I, Zhongliang W . Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis 2015; 2: 13–25.
Gazdar AF . Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene 2009; 28: S24–S31.
Martinelli E, Palma RD, Orditura M, Vita FD, Ciardiello F . Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol 2009; 158: 1–9.
Khelwatty SA, Essapen S, Seddon AM, Fan Z, Modjtahedi H . Acquired resistance to anti-EGFR mAb ICR62 in cancer cells is accompanied by an increased EGFR expression, HER-2/HER-3 signalling and sensitivity to pan HER blockers. Br J Cancer 2015; 113: 1010–1019.
Alejandra T, Clare EF, Emily RE . EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol 2014; 24: 26–34.
Li L, Puliyappadamba VT, Chakraborty S, Rehman A, Vemireddy V . EGFR wild type antagonizes EGFRvIII-mediated activation of Met in glioblastoma. Oncogene 2015; 34: 129–134.
Shi J, Li YJ, Yan B, Wei PK . Interleukin-8: A potent promoter of human lymphatic endothelial cell growth in gastric cancer. Oncol Rep 2015; 33: 2703–2710.
Lu Y, Li X, Liang K, Luwor R, Siddik ZH, Mills GB et al. Epidermal growth factor receptor (EGFR) ubiquitination as a mechanism of acquired resistance escaping treatment by the anti-EGFR monoclonal antibody cetuximab. Cancer Res 2007; 67: 8240–8247.
Erika M, Floriana M, Teresa T, Giampaolo T . Panitumumab: the evidence of its therapeutic potential in metastatic colorectal cancer care. Core Evid 2007; 2: 81–88.
Daoud MA, Aboelnaga EM, Mohamed WM . Second-line panitumumab as a triweekly dose for patients with wild-type KRAS exon 2 metastatic colorectal cancer: a single-institution experience. Cancer Biol Med 2016; 13: 136–141.
Schmitt DA, Bieber T, Cazenave JP, Hanau D . Fc receptors of human Langerhans cells. J Invest Dermatol 1990; 94: 15S–21S.
Tuijnman WB, Van Wichen DF, Schuurman HJ . Tissue distribution of human IgG Fc receptors CD16, CD32 and CD64: an immune histochemical study. APMIS 1993; 101: 319–329.
Li X, Kimberly RP . Targeting the Fc receptor in autoimmune disease. Expert Opin Ther Targets 2014; 18: 335–350.
Sánchez FJ, Bellosillo B, Gelabert BM, Dalmases A, Cañadas I et al. The first-in-class Anti-EGFR Antibody Mixture Sym004 overcomes cetuximab resistance mediated by egfr extracellular domain mutations in colorectal cancer. Clin Cancer Res 2016; 22: 3260–3267.
Arena S, Siravegna G, Mussolin B, Kearns JD, Wolf BB, Misale S . MM-151 overcomes acquired resistance to cetuximab and panitumumab in colorectal cancers harboring EGFR extracellular domain mutations. Sci Transl Med 2016; 8: 324–331.
Acknowledgements
We are thankful to the Department of Biotechnology (Grant Number GAP0405) and Council of Scientific and Industrial Research (Grant Number BSC0208) and AB SCIEX, India for funding and help. We are grateful to all the lab members for fruitful discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dokala, A., Thakur, S. Extracellular region of epidermal growth factor receptor: a potential target for anti-EGFR drug discovery. Oncogene 36, 2337–2344 (2017). https://doi.org/10.1038/onc.2016.393
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.393
This article is cited by
-
Conformational diversity and protein–protein interfaces in drug repurposing in Ras signaling pathway
Scientific Reports (2024)
-
Clinical perspectives and outcomes of the giant breast phyllodes tumor and sarcoma: a real-world retrospective study
BMC Cancer (2023)
-
Antibody-drug conjugates in lung cancer: dawn of a new era?
npj Precision Oncology (2023)
-
Dual-responsive nanoparticles loading bevacizumab and gefitinib for molecular targeted therapy against non-small cell lung cancer
Acta Pharmacologica Sinica (2023)
-
Sorcin promotes migration in cancer and regulates the EGF-dependent EGFR signaling pathways
Cellular and Molecular Life Sciences (2023)