Key Points
-
The propensity for kidney stone formation in patients who have undergone bariatric surgery is well documented and presents a unique treatment challenge
-
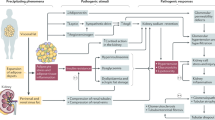
The pathogenesis of kidney stones in these patients involves the malabsorption of fat, which leads to unbound oxalate being absorbed in excess in the gut
-
Specific 24 h urine derangements in the bariatric surgery population include hyperoxaluria, hyperuricosuria, low urine volume and hypocitraturia
-
Dietary prevention of stones with a high-fluid diet and limitation of oxalate and sodium is key; calcium supplementation should also be considered
-
Potassium citrate is helpful as an alkalinizing agent and promotes activity of stone inhibitors
-
Pyridoxine and probiotics require further investigation in this setting
Abstract
Obesity is a significant health concern and is associated with an increased risk of nephrolithiasis, particularly in women. The underlying pathophysiology of stone formation in obese patients is thought to be related to insulin resistance, dietary factors, and a lithogenic urinary profile. Uric acid stones and calcium oxalate stones are common in these patients. Use of surgical procedures for obesity (bariatric surgery) has risen over the past two decades. Although such procedures effectively manage obesity-dependent comorbidities, several large, controlled studies have revealed that modern bariatric surgeries increase the risk of nephrolithiasis by approximately twofold. In patients who have undergone bariatric surgery, fat malabsorption leads to hyperabsorption of oxalate, which is exacerbated by an increased permeability of the gut to oxalate. Patients who have undergone bariatric surgery show characteristic 24 h urine parameters including low urine volume, low urinary pH, hypocitraturia, hyperoxaluria and hyperuricosuria. Prevention of stones with dietary limitation of oxalate and sodium and a high intake of fluids is critical, and calcium supplementation with calcium citrate is typically required. Potassium citrate is valuable for treating the common metabolic derangements as it raises urinary pH, enhances the activity of stone inhibitors, reduces the supersaturation of calcium oxalate, and corrects hypokalaemia. Both pyridoxine and probiotics have been shown in small studies to reduce hyperoxaluria, but further study is necessary to clarify their effects on stone morbidity in the bariatric surgery population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ogden, C. L., Carroll, M. D., Kit, B. K. & Flegal, K. M. Prevalence of childhood and adult obesity in the United States, 2011–2012 JAMA 311, 806–814 (2014).
DeMaria, E. J. Bariatric surgery for morbid obesity. N. Engl. J. Med. 356, 2176–2183 (2007).
Nguyen, N. T. et al. Trends in use of bariatric surgery, 2003−2008. J. Am. Coll. Surg. 213, 261–266 (2011).
Davis, M. M., Slish, K., Chao, C. & Cabana, M. D. National trends in bariatric surgery, 1996–2002 Arch. Surg. 141, 71–74 (2006).
Buchwald, H. & Oien, D. M. Metabolic/bariatric surgery worldwide 2011. Obes. Surg. 23, 427–436 (2013).
Nelson, W. K., Houghton, S. G., Milliner, D. S., Lieske, J. C. & Sarr, M. G. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 1, 481–485 (2005).
Lieske, J. C. et al. Kidney stones are common after bariatric surgery. Kidney Int. http://dx.doi.org/10.1038/ki.2014.352.
Matlaga, B. R. et al. Effect of gastric bypass surgery on kidney stone disease. J. Urol. 181, 2573–2577 (2009).
Gonzalez, R. D. & Canales, B. K. Kidney stone risk following modern bariatric surgery. Curr. Urol. Rep. 15, 401 (2014).
Duffey, B. G. et al. Roux-en-Y gastric bypass is associated with early increased risk factors for development of calcium oxalate nephrolithiasis. J. Am. Coll. Surg. 206, 1145–1153 (2008).
Taylor, E. N., et al. Obesity, weight gain, and the risk of kidney stones. JAMA 293, 455–462 (2005).
Sakhaee, K., Adams-Huet, B., Moe, O. W. & Pak, C. Y. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 62, 971–979 (2002).
Taylor, E. N., Stampfer, M. J. & Curhan, G. C. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 68, 1230–1235 (2005).
Sakhaee, K. Nephrolithiasis as a systemic disorder. Curr. Opin. Nephrol. Hypertens. 17, 304–309 (2008).
Cameron, M. A., Maalouf, N. M., Adams-Huet, B., Moe, O. W. & Sakhaee, K. Urine composition in type 2 diabetes: predisposition to uric acid nephrolithiasis. J. Am. Soc. Nephrol. 17, 1422–1428 (2006).
Maalouf, N. M., Cameron, M. A., Moe, O. W., Adams-Huet, B. & Sakhaee, K. Low urine pH: a novel feature of the metabolic syndrome. Clin. J. Am. Soc. Nephrol. 2, 883–888 (2007).
Abate, N., Chandalia, M., Cabo-Chan, A. V. Jr, Moe, O. W. & Sakhaee, K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 65, 386–392 (2004).
Chen, T., Godebu, E., Horgan, S., Mirheydar, H. S. & Sur, R. L. The effect of restrictive bariatric surgery on urolithiasis. J. Endourol. 27, 242–244 (2013).
Penniston, K. L., Kaplon, D. M., Gould, J. C. & Nakada, S. Y. Gastric band placement for obesity is not associated with increased urinary risk of urolithiasis compared to bypass. J. Urol. 182, 2340–2346 (2009).
Duffey, B. G. et al. Hyperoxaluria is a long-term consequence of Roux-en-Y Gastric bypass: a 2-year prospective longitudinal study. J. Am. Coll. Surg. 211, 8–15 (2010).
Maalouf, N. M., Tondapu, P., Guth, E. S., Livingston, E. H. & Sakhaee, K. Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J. Urol. 183, 1026–1030 (2010).
Wein, A. J., Kavoussi, L. R. & Campbell, M. F. in Campbell–Walsh Urology 10th edn (eds Wein, A. J., Kavoussi, L. R., Novick, A. C., Partin, A. W. & Peters, C. A.) 1287–1323 (Saunders, 2012).
Kumar, R. et al. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery 149, 654–661 (2011).
Streem, S. B. Long-term incidence and risk factors for recurrent stones following percutaneous nephrostolithotomy or percutaneous nephrostolithotomy/extracorporeal shock wave lithotripsy for infection related calculi. J. Urol. 153, 584–587 (1995).
Teichman, J. M., Long, R. D. & Hulbert, J. C. Long-term renal fate and prognosis after staghorn calculus management. J. Urol. 153, 1403–1407 (1995).
Vupputuri, S., Soucie, J. M., McClellan, W. & Sandler, D. P. History of kidney stones as a possible risk factor for chronic kidney disease. Ann. Epidemiol. 14, 222–228 (2004).
[No authors listed] Incidence and prevalence of ESRD. United States Renal Data System. Am. J. Kidney Dis. 32 (Suppl. 1), S38–S49 (1998).
Powell, C. R. et al. Impact of body weight on urinary electrolytes in urinary stone formers. Urology 55, 825–830 (2000).
Negri, A. L. et al. Role of overweight and obesity on the urinary excretion of promoters and inhibitors of stone formation in stone formers. Urol. Res. 36, 303–307 (2008).
Maalouf, N. M. et al. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 65, 1422–1425 (2004).
Ekeruo, W. O. et al. Metabolic risk factors and the impact of medical therapy on the management of nephrolithiasis in obese patients. J. Urol. 172, 159–163 (2004).
Pigna, F., Sakhaee, K., Adams-Huet, B. & Maalouf, N. M. Body fat content and distribution and urinary risk factors for nephrolithiasis. Clin. J. Am. Soc. Nephrol. 9, 159–165 (2014).
Del Valle, E. E. et al. Metabolic diagnosis in stone formers in relation to body mass index. Urol. Res. 40, 47–52 (2012).
Asplin, J. R. & Coe, F. L. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J. Urol. 177, 565–569 (2007).
Sinha, M. et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 72, 100–107 (2007).
Valezi, A. C., Fuganti, P. E., Junior, J. M. & Delfino, V. D. Urinary evaluation after RYGBP: a lithogenic profile with early postoperative increase in the incidence of urolithiasis. Obes. Surg. 23, 1575–1580 (2013).
Semins, M. J. et al. The effect of restrictive bariatric surgery on urinary stone risk factors. Urology 76, 826–829 (2010).
Colquitt, J. L., Pickett, K., Loveman, E. & Frampton, G. K. Surgery for weight loss in adults. Cochrane Database of Systematic Reviews, Issue 8. Art. No.: CD003641 http://dx.doi.org/10.1002/14651858.CD003641.pub4.
Borghi, L. et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N. Engl. J. Med. 346, 77–84 (2002).
Curhan, G. C., Willett, W. C., Speizer, F. E., Spiegelman, D. & Stampfer, M. J. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann. Intern. Med. 126, 497–504 (1997).
Sakhaee, K. et al. Limited risk of kidney stone formation during long-term calcium citrate supplementation in nonstone forming subjects. J. Urol. 152, 324–327 (1994).
Tondapu, P. et al. Comparison of the absorption of calcium carbonate and calcium citrate after Roux-en-Y gastric bypass. Obes. Surg. 19, 1256–1261 (2009).
Froeder, L., Arasaki, C. H., Malheiros, C. A., Baxmann, A. C. & Heilberg, I. P. Response to dietary oxalate after bariatric surgery. Clin. J. Am. Soc. Nephrol. 7, 2033–2040 (2012).
Xu, H., Zisman, A. L., Coe, F. L. & Worcester, E. M. Kidney stones: an update on current pharmacological management and future directions. Expert Opin. Pharmacother. 14, 435–447 (2013).
Sakhaee, K., Harvey, J. A., Padalino, P. K., Whitson, P. & Pak, C. Y. The potential role of salt abuse on the risk for kidney stone formation. J. Urol. 150, 310–312 (1993).
Burns, J. & Finlayson, B. Strategies for the medical management of patients with urinary stone disease. Monogr. Urol. 2, 106–125 (1981).
Caudarella, R. et al. Renal stone formation in patients with inflammatory bowel disease. Scanning Microsc. 7, 371–379 (1993).
Ettinger, B., Citron, J., Livermore, B. & Dolman, L. Chlorthalidone reduces calcium oxalate calculous recurrence but magnesium hydroxide does not. J. Urol. 139, 679–684 (1988).
Pearle, M. S. et al. Medical management of kidney stones: AUA guideline. J. Urol. 192, 316–324 (2014).
Sakhaee, K., Griffith, C. & Pak, C. Y. Biochemical control of bone loss and stone-forming propensity by potassium-calcium citrate after bariatric surgery. Surg. Obes. Relat. Dis. 8, 67–72 (2012).
Jaeger, P., Portmann, L., Jacquet, A. F. & Burckhardt, P. Pyridoxine can normalize oxaluria in idiopathic renal lithiasis [French]. Schweiz Med. Wochenschr. 116, 1783–1786 (1986).
Mitwalli, A., Ayiomamitis, A., Grass, L. & Oreopoulos, D. G. Control of hyperoxaluria with large doses of pyridoxine in patients with kidney stones. Int. Urol. Nephrol. 20, 353–359 (1988).
Bobrowski, A. E. & Langman, C. B. Hyperoxaluria and systemic oxalosis: current therapy and future directions. Expert Opin. Pharmacother. 7, 1887–1896 (2006).
Hatch, M. et al. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int. 69, 691–698 (2006).
Sidhu, H., Allison, M. J., Chow, J. M., Clark, A. & Peck, A. B. Rapid reversal of hyperoxaluria in a rat model after probiotic administration of Oxalobacter formigenes. J. Urol. 166, 1487–1491 (2001).
Lieske, J. C., Goldfarb, D. S., De Simone, C. & Regnier, C. Use of a probioitic to decrease enteric hyperoxaluria. Kidney Int. 68, 1244–1249 (2005).
Author information
Authors and Affiliations
Contributions
S.T. and V.G. researched data for the article and wrote the article. S.T. and M.M. provided a substantial contribution to the discussion of content for the article and reviewed/edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tarplin, S., Ganesan, V. & Monga, M. Stone formation and management after bariatric surgery. Nat Rev Urol 12, 263–270 (2015). https://doi.org/10.1038/nrurol.2015.67
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2015.67
This article is cited by
-
Micronutrient inadequacy and urinary stone disease: an analysis of the National Health and Nutrition Examination Survey 2007–2018
Urolithiasis (2023)
-
Dietary advice for patients with bowel-related conditions and malabsorption
World Journal of Urology (2023)
-
The Short-Term Renal Effects of Bariatric Surgery: A Comparative Study Between Sleeve Gastrectomy and One Anastomosis Gastric Bypass Operations Among Egyptian Patients With Severe Obesity
Obesity Surgery (2020)
-
Nachsorge nach bariatrischer Chirurgie
Der Diabetologe (2015)