Key Points
-
The risk of ischaemic stroke is associated with systemic inflammation and inflammatory biomarkers, suggesting a causal relationship
-
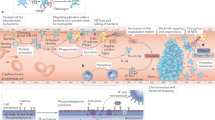
Acute infections activate the inflammatory cascade, which might increase the subsequent risk of stroke
-
Chronic infection and infectious burden have been associated with an increased risk of stroke
-
Various inflammatory mechanisms probably have different roles in different subtypes of stroke
-
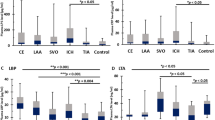
C-Reactive protein, IL-6 and lipoprotein-associated phospholipase A2 are indicators of chronic inflammation and might be biomarkers of stroke risk
-
Various general and targeted therapeutic strategies that aim to decrease the risk of stroke by reducing inflammation have been studied and remain under investigation
Abstract
Proinflammatory conditions, including acute and chronic infections, have been associated with an increased risk of stroke. The risk of stroke is increased by both the acute and chronic phases of a wide spectrum of proinflammatory conditions, suggesting that the association is related to activation of the inflammatory response rather than the condition itself. Different inflammatory mechanisms probably influence the risk of different stroke subtypes. This hypothesis is supported by observations that high levels of various immune system markers and acute phase reactants in otherwise healthy individuals have also been associated with ischaemic stroke subtypes. C-reactive protein, IL-6 and lipoprotein-associated phospholipase A2 are some of the inflammatory markers that have been associated with stroke risk and prognosis. Multiple epidemiological studies have demonstrated that these markers are associated with the risk of stroke, but the value of these markers in a clinical setting has not yet been proven. Further research is needed to determine whether immune system modulators can lower the risk of stroke in individuals with elevated concentrations of inflammatory markers. Here, we review the association between infection, systemic inflammation, and ischaemic stroke, and discuss the currently recommended preventive methods to decrease the risk of stroke associated with systemic inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mendis, S., Davis, S. & Norrving, B. Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 46, e121–122 (2015).
Adams, H. P. Jr et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24, 35–41 (1993).
Litman, G. W., Rast, J. P. & Fugmann, S. D. The origins of vertebrate adaptive immunity. Nat. Rev. Immunol. 10, 543–553 (2010).
Hansson, G. K. & Berne, G. P. Atherosclerosis and the immune system. Acta Paediatr. Suppl. 93, 63–69 (2004).
Opal, S. M. & Esmon, C. T. Bench-to-bedside review: functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Crit. Care 7, 23–38 (2003).
Delvaeye, M. & Conway, E. M. Coagulation and innate immune responses: can we view them separately? Blood 114, 2367–2374 (2009).
Ross, R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999). An overview of the inflammatory mechanisms underlying atherogenesis.
Antoniak, S. & Mackman, N. Multiple roles of the coagulation protease cascade during virus infection. Blood 123, 2605–2613 (2014).
Russell, J. A. Management of sepsis. N. Engl. J. Med. 355, 1699–1713 (2006).
Wang, R., Xiao, H., Guo, R., Li, Y. & Shen, B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg. Microbes Infect. 4, e28 (2015).
Cermak, J. et al. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood 82, 513–520 (1993).
Wu, J. et al. C-reactive protein enhances tissue factor expression by vascular smooth muscle cells: mechanisms and in vivo significance. Arterioscler. Thromb. Vasc. Biol. 28, 698–704 (2008).
Elkind, M. S. et al. Hospitalization for infection and risk of acute ischemic stroke: the Cardiovascular Health Study. Stroke 42, 1851–1856 (2011). This article provides evidence that acute infection is associated with a subsequent period of increased stroke risk.
Levine, D. A., Langa, K. M. & Rogers, M. A. Acute infection contributes to racial disparities in stroke mortality. Neurology 82, 914–921 (2014).
Paganini-Hill, A. et al. Infection and risk of ischemic stroke: differences among stroke subtypes. Stroke 34, 452–457 (2003). This study suggests that acute respiratory infections lead to higher risk of stroke from large vessel atherosclerosis and cardioembolism.
Selvarajah, J. R. et al. Does inflammation predispose to recurrent vascular events after recent transient ischaemic attack and minor stroke? The north-west of England transient ischaemic attack and minor stroke (NORTHSTAR) Study. Int. J. Stroke 6, 187–194 (2011).
Vlachopoulos, C. et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation 112, 2193–2200 (2005).
Grau, A. J. et al. Leukocyte count as an independent predictor of recurrent ischemic events. Stroke 35, 1147–1152 (2004).
Espinola-Klein, C. et al. Are morphological or functional changes in the carotid artery wall associated with Chlamydia pneumoniae. Helicobacter pylori, cytomegalovirus, or herpes simplex virus infection? Stroke 31, 2127–2133 (2000).
Sorlie, P. D. et al. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Arch. Intern. Med. 160, 2027–2032 (2000).
Kalayoglu, M. V., Libby, P. & Byrne, G. I. Chlamydia pneumoniae as an emerging risk factor in cardiovascular disease. JAMA 288, 2724–2731 (2002).
Elkind, M. S. et al. Infectious burden and risk of stroke: the Northern Manhattan Study. Arch. Neurol. 67, 33–38 (2010).
Elkind, M. S. Infectious burden: a new risk factor and treatment target for atherosclerosis. Infect. Disord. Drug Targets 10, 84–90 (2010).
Smieja, M. et al. Multiple infections and subsequent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation 107, 251–257 (2003).
Katan, M. et al. Infectious burden and cognitive function: the Northern Manhattan Study. Neurology 80, 1209–1215 (2013).
Kiechl, S. et al. Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population study. Circulation 103, 1064–1070 (2001).
Palm, F. et al. Biomarkers of periodontitis and inflammation in ischemic stroke: a case-control study. Innate Immun. 20, 511–518 (2014).
Grau, A. J. et al. Association of symptoms of chronic bronchitis and frequent flu-like illnesses with stroke. Stroke 40, 3206–3210 (2009).
Sfyroeras, G. S., Roussas, N., Saleptsis, V. G., Argyriou, C. & Giannoukas, A. D. Association between periodontal disease and stroke. J. Vasc. Surg. 55, 1178–1184 (2012).
Haider, A. W. et al. The association of seropositivity to Helicobacter pylori. Chlamydia pneumoniae, and cytomegalovirus with risk of cardiovascular disease: a prospective study. J. Am. Coll. Cardiol. 40, 1408–1413 (2002).
Aiello, A. E. et al. Socioeconomic and psychosocial gradients in cardiovascular pathogen burden and immune response: the multi-ethnic study of atherosclerosis. Brain Behav. Immun. 23, 663–671 (2009).
Nagel, M. A. et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 77, 364–370 (2011).
Sico, J. J. et al. HIV status and the risk of ischemic stroke among men. Neurology 84, 1933–1940 (2015).
Islam, F. M., Wu, J., Jansson, J. & Wilson, D. P. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 13, 453–468 (2012).
Gutierrez, J. et al. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV. Neurology 85, 1139–1145 (2015).
Di Napoli, M. & Papa, F. Inflammation, hemostatic markers, and antithrombotic agents in relation to long-term risk of new cardiovascular events in first-ever ischemic stroke patients. Stroke 33, 1763–1771 (2002).
Pearson, T. A. et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107, 499–511 (2003).
Ridker, P. M., Cushman, M., Stampfer, M. J., Tracy, R. P. & Hennekens, C. H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 336, 973–979 (1997).
Ridker, P. M., Rifai, N., Rose, L., Buring, J. E. & Cook, N. R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 347, 1557–1565 (2002). This study provides evidence that hsCRP is a strong independent predictor of first-time stroke.
Ridker, P. M., Buring, J. E., Shih, J., Matias, M. & Hennekens, C. H. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 98, 731–733 (1998).
Salazar, J. et al. C-reactive protein: clinical and epidemiological perspectives. Cardiol. Res. Pract. 2014, 605810 (2014).
Ji, S. R. et al. Cell membranes and liposomes dissociate C-reactive protein (CRP) to form a new, biologically active structural intermediate: mCRP(m). FASEB J. 21, 284–294 (2007).
Eisenhardt, S. U. et al. Dissociation of pentameric to monomeric C-reactive protein on activated platelets localizes inflammation to atherosclerotic plaques. Circ. Res. 105, 128–137 (2009).
Shrive, A. K. et al. Three dimensional structure of human C-reactive protein. Nat. Struct. Biol. 3, 346–354 (1996).
Thompson, D., Pepys, M. B. & Wood, S. P. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure 7, 169–177 (1999).
Fujita, Y. et al. Oxidized LDL receptor LOX-1 binds to C-reactive protein and mediates its vascular effects. Clin. Chem. 55, 285–294 (2009).
Fujita, Y. et al. Lectin-like oxidized LDL receptor 1 is involved in CRP-mediated complement activation. Clin. Chem. 57, 1398–1405 (2011).
Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 2045–2051 (2012).
The Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 375, 132–140 (2010). This meta-analysis provides strong evidence of the association between hsCRP and stroke.
Zhou, Y., Han, W., Gong, D., Man, C. & Fan, Y. Hs-CRP in stroke: a meta-analysis. Clin. Chim. Acta 453, 21–27 (2016).
Curb, J. D. et al. C-reactive protein and the future risk of thromboembolic stroke in healthy men. Circulation 107, 2016–2020 (2003).
Elkind, M. S. et al. High-sensitivity C-reactive protein predicts mortality but not stroke: the Northern Manhattan Study. Neurology 73, 1300–1307 (2009).
Luna, J. M. et al. High-sensitivity C-reactive protein and interleukin-6-dominant inflammation and ischemic stroke risk the Northern Manhattan Study. Stroke 45, 979–987 (2014).
van Dijk, E. J. et al. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation 112, 900–905 (2005).
Bos, M. J. et al. High serum C-reactive protein level is not an independent predictor for stroke: the Rotterdam Study. Circulation 114, 1591–1598 (2006).
Zacho, J. et al. Genetically elevated C-reactive protein and ischemic vascular disease. N. Engl. J. Med. 359, 1897–1908 (2008).
Ridker, P. M. et al. Loci related to metabolic-syndrome pathways including LEPR,HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women's Genome Health Study. Am. J. Hum. Genet. 82, 1185–1192 (2008).
Fornage, M. et al. Biomarkers of inflammation and MRI-defined small vessel disease of the brain: the Cardiovascular Health Study. Stroke 39, 1952–1959 (2008).
Elkind, M. S., Tai, W., Coates, K., Paik, M. C. & Sacco, R. L. High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch. Intern. Med. 166, 2073–2080 (2006).
Woodward, M. et al. Associations of inflammatory and hemostatic variables with the risk of recurrent stroke. Stroke 36, 2143–2147 (2005).
Welsh, P. et al. Associations of proinflammatory cytokines with the risk of recurrent stroke. Stroke 39, 2226–2230 (2008).
Elkind, M. S. V. et al. C-reactive protein as a prognostic marker after lacunar stroke: levels of inflammatory markers in treatment of stroke. Stroke 45, 707–716 (2014). This study provides evidence from a multicenter stroke trial of the association between hsCRP and recurrent stroke among lacunar stroke patients.
Schuett, H., Luchtefeld, M., Grothusen, C., Grote, K. & Schieffer, B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb. Haemost. 102, 215–222 (2009).
C Reactive Protein Coronary Heart Disease Genetics Collaboration (CCGC). Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ 342, d548 (2011).
IL6R Genetics Consortium Emerging Risk Factors Collaboration. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 379, 1205–1213 (2012).
Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 379, 1214–1224 (2012). This Mendelian randomization analysis suggests a causal association between IL-6 and cardiovascular disease.
Kaptoge, S. et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur. Heart J. 35, 578–589 (2014).
Patterson, C. C. et al. The associations of interleukin-6 (IL-6) and downstream inflammatory markers with risk of cardiovascular disease: the Caerphilly Study. Atherosclerosis 209, 551–557 (2010).
Solomon, D. H. et al. Cardiovascular risk in rheumatoid arthritis: comparing TNF-α blockade with nonbiologic DMARDs. Am. J. Med. 126, 730.e9–730.e17 (2013).
Marks, J. L. & Edwards, C. J. Protective effect of methotrexate in patients with rheumatoid arthritis and cardiovascular comorbidity. Ther. Adv. Musculoskelet. Dis. 4, 149–157 (2012).
Rosenson, R. S. & Stafforini, D. M. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2 . J. Lipid Res. 53, 1767–1782 (2012).
Oei, H. H. et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation 111, 570–575 (2005).
Katan, M. et al. Lipoprotein-associated phospholipase A2 is associated with atherosclerotic stroke risk: the Northern Manhattan Study. PLoS ONE 9, e83393 (2014).
Elkind, M. S., Tai, W., Coates, K., Paik, M. C. & Sacco, R. L. Lipoprotein-associated phospholipase A2 activity and risk of recurrent stroke. Cerebrovasc. Dis. 27, 42–50 (2009).
Ballantyne, C. M. et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) Study. Arch. Intern. Med. 165, 2479–2484 (2005).
Ridker, P. M., MacFadyen, J. G., Wolfert, R. L. & Koenig, W. Relationship of lipoprotein-associated phospholipase A2 mass and activity with incident vascular events among primary prevention patients allocated to placebo or to statin therapy: an analysis from the JUPITER trial. Clin. Chem. 58, 877–886 (2012).
Stein, E. A. Lipoprotein-associated phospholipase A2 measurements: mass, activity, but little productivity. Clin. Chem. 58, 814–817 (2012).
Mohler, E. R. et al. The effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity and cardiovascular biomarkers in patients with stable coronary heart disease or coronary heart disease risk equivalent: the results of a multicenter, randomized, double-blind, placebo-controlled study. J. Am. Coll. Cardiol. 51, 1632–1641 (2008).
O'Donoghue, M. L. et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA 312, 1006–1015 (2014).
The Lp-PLA2 Studies Collaboration. Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet 375, 1536–1544 (2010). This article includes a meta-analysis that supports the association between LP-PLa2 and stroke.
Schillinger, M. et al. Inflammation and Carotid Artery—Risk for Atherosclerosis Study (ICARAS). Circulation 111, 2203–2209 (2005).
Elkind, M. S. et al. Tumor necrosis factor receptor levels are associated with carotid atherosclerosis. Stroke 33, 31–37 (2002).
Kolominsky-Rabas, P. L., Weber, M., Gefeller, O., Neundoerfer, B. & Heuschmann, P. U. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 32, 2735–2740 (2001).
White, H. et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 111, 1327–1331 (2005).
Warlow, C. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet 337, 1235–1243 (1991).
North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 325, 445–453 (1991).
Cao, J. J. et al. C-reactive protein, carotid intima-media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation 108, 166–170 (2003).
Alvarez Garcia, B., Ruiz, C., Chacon, P., Sabin, J. A. & Matas, M. High-sensitivity C-reactive protein in high-grade carotid stenosis: risk marker for unstable carotid plaque. J. Vasc. Surg. 38, 1018–1024 (2003).
Mauriello, A. et al. A pathobiologic link between risk factors profile and morphological markers of carotid instability. Atherosclerosis 208, 572–580 (2010).
Rizzo, M. et al. Prediction of cardio- and cerebro-vascular events in patients with subclinical carotid atherosclerosis and low HDL-cholesterol. Atherosclerosis 200, 389–395 (2008).
Belfer, I. et al. Linkage of large-vessel carotid atherosclerotic stroke to inflammatory genes via a systematic screen. Int. J. Stroke 5, 145–151 (2010).
Wong, L. K. Global burden of intracranial atherosclerosis. Int. J. Stroke 1, 158–159 (2006).
Chimowitz, M. I. et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N. Engl. J. Med. 365, 993–1003 (2011).
Arenillas, J. F. et al. Progression of symptomatic intracranial large artery atherosclerosis is associated with a proinflammatory state and impaired fibrinolysis. Stroke 39, 1456–1463 (2008).
Miwa, K. et al. Association between interleukin-6 levels and first-ever cerebrovascular events in patients with vascular risk factors. Arterioscler. Thromb. Vasc. Biol. 33, 400–405 (2013).
Hoshi, T. et al. Relations of serum high-sensitivity C-reactive protein and interleukin-6 levels with silent brain infarction. Stroke 36, 768–772 (2005).
Reganon, E. et al. Association between inflammation and hemostatic markers in atherothrombotic stroke. Thromb. Res. 112, 217–221 (2003).
Satizabal, C. L., Zhu, Y. C., Mazoyer, B., Dufouil, C. & Tzourio, C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology 78, 720–727 (2012).
Wright, C. B. et al. Inflammatory biomarkers of vascular risk as correlates of leukoariosis. Stroke 40, 3466–3471 (2009).
Rouhl, R. P. et al. Vascular inflammation in cerebral small vessel disease. Neurobiol. Aging 33, 1800–1806 (2012).
Gao, S. P. et al. Is inflammation linked to thrombogenesis in atrial fibrillation? Int. J. Cardiol. 149, 260–261 (2011).
Marott, S. C. et al. Does elevated C-reactive protein increase atrial fibrillation risk? A Mendelian randomization of 47,000 individuals from the general population. J. Am. Coll. Cardiol. 56, 789–795 (2010).
Chung, M. K. et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 104, 2886–2891 (2001).
Conway, D. S., Buggins, P., Hughes, E. & Lip, G. Y. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J. Am. Coll. Cardiol. 43, 2075–2082 (2004).
Ederhy, S. et al. C-reactive protein and transesophageal echocardiographic markers of thromboembolism in patients with atrial fibrillation. Int. J. Cardiol. 159, 40–46 (2012).
Kamel, H. et al. Electrocardiographic left atrial abnormality and risk of stroke: Northern Manhattan Study. Stroke 46, 3208–3212 (2015).
Kamel, H., Okin, P., Elkind, M. S. & Iadecola, C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke 47, 895–900 (2016).
Ford, E. S. Does exercise reduce inflammation? Physical activity and C-reactive protein among U. S. adults. Epidemiology 13, 561–568 (2002).
Sacco, R. L. et al. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke 29, 380–387 (1998).
Lee, I. M., Hennekens, C. H., Berger, K., Buring, J. E. & Manson, J. E. Exercise and risk of stroke in male physicians. Stroke 30, 1–6 (1999).
Esposito, K. et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 292, 1440–1446 (2004).
Serrano-Martinez, M. et al. A Mediterranean dietary style influences TNF-α and VCAM-1 coronary blood levels in unstable angina patients. Eur. J. Nutr. 44, 348–354 (2005).
Salas-Salvado, J. et al. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur. J. Clin. Nutr. 62, 651–659 (2008).
Lopez-Garcia, E. et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 80, 1029–1035 (2004).
Estruch, R. et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 368, 1279–1290 (2013).
Gao, X., Bermudez, O. I. & Tucker, K. L. Plasma C-reactive protein and homocysteine concentrations are related to frequent fruit and vegetable intake in Hispanic and non-Hispanic white elders. J. Nutr. 134, 913–918 (2004).
Nichol, K. L. et al. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N. Engl. J. Med. 348, 1322–1332 (2003).
Clar, C., Oseni, Z., Flowers, N., Keshtkar-Jahromi, M. & Rees, K. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst. Rev. 5, CD005050 (2015).
Smeeth, L. et al. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 351, 2611–2618 (2004).
Lavallée, P., Perchaud, V., Gautier-Bertrand, M., Grabli, D. & Amarenco, P. Association between influenza vaccination and reduced risk of brain infarction. Stroke 33, 513–518 (2002). This article recommends influenza vaccination to prevent secondary cardiovascular risk, and reviews the evidence for this recommendation.
Davis, M. M. et al. Influenza vaccination as secondary prevention for cardiovascular disease: a science advisory from the American Heart Association/American College of Cardiology. Circulation 114, 1549–1553 (2006).
Gurfinkel, E. P., de la Fuente, R. L., Mendiz, O. & Mautner, B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions: the FLU Vaccination Acute Coronary Syndromes (FLUVACS) Study. Circulation 105, 2143–2147 (2002).
Tseng, H. F. et al. Pneumococcal vaccination and risk of acute myocardial infarction and stroke in men. JAMA 303, 1699–1706 (2010).
Wolf, D., Zirlik, A. & Ley, K. Beyond vascular inflammation-recent advances in understanding atherosclerosis. Cell. Mol. Life Sci. 72, 3853–3869 (2015).
Cannon, C. P. et al. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N. Engl. J. Med. 352, 1646–1654 (2005).
Grayston, J. T. et al. Azithromycin for the secondary prevention of coronary events. N. Engl. J. Med. 352, 1637–1645 (2005).
Andraws, R., Berger, J. S. & Brown, D. L. Effects of antibiotic therapy on outcomes of patients with coronary artery disease: a meta-analysis of randomized controlled trials. JAMA 293, 2641–2647 (2005). This meta-analysis concludes that primary prevention treatment with rosuvastatin in high-risk patients stratified using hsCRP levels can improve outcomes.
Ridker, P. M. et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359, 2195–2207 (2008).
Davignon, J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 109, (Suppl. 1) III-39–III-43 (2004).
Chen, H., Ikeda, U., Shimpo, M. & Shimada, K. Direct effects of statins on cells primarily involved in atherosclerosis. Hypertens. Res. 23, 187–192 (2000).
Comparato, C. et al. Clinically relevant pleiotropic effects of statins: drug properties or effects of profound cholesterol reduction? Nutr. Metab. Cardiovasc. Dis. 11, 328–343 (2001).
Rasmussen, L. M. et al. Diverse effects of inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase on the expression of VCAM-1 and E-selectin in endothelial cells. Biochem. J. 360, 363–370 (2001).
Hurlimann, D. et al. Anti-tumor necrosis factor-α treatment improves endothelial function in patients with rheumatoid arthritis. Circulation 106, 2184–2187 (2002).
Rayment, N. B. et al. Synthesis of TNFα and TGFβ mRNA in the different micro-environments within atheromatous plaques. Cardiovasc. Res. 32, 1123–1130 (1996).
Ridker, P. M. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT). J. Thromb. Haemost. 7 (Suppl. 1), 332–339 (2009).
Ridker, P. M., Thuren, T., Zalewski, A. & Libby, P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 162, 597–605 (2011).
Salminen, A. & Kaarniranta, K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 11, 230–241 (2012).
Ricote, M., Li, A. C., Willson, T. M., Kelly, C. J. & Glass, C. K. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 391, 79–82 (1998).
Li, A. C. et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ. J. Clin. Invest. 114, 1564–1576 (2004).
Zelvyte, I., Dominaitiene, R., Crisby, M. & Janciauskiene, S. Modulation of inflammatory mediators and PPARγ and NFκB expression by pravastatin in response to lipoproteins in human monocytes in vitro. Pharmacol. Res. 45, 147–154 (2002).
White, G. E., Iqbal, A. J. & Greaves, D. R. CC chemokine receptors and chronic inflammation—therapeutic opportunities and pharmacological challenges. Pharmacol. Rev. 65, 47–89 (2013).
Kleinbongard, P., Heusch, G. & Schulz, R. TNFα in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol. Ther. 127, 295–314 (2010).
Acknowledgements
The authors acknowledge funding support for work described in this review from the National Institute of Neurological Disorders and Stroke (NINDS R01 NS29993, R01 NS050724, T32 NS07153); the Bristol-Myers Squibb–Sanofi Partnership and diaDexus; and the American Heart Association (Grant-in-Aid 0355596T; Kathleen Scott Fellowship).
Author information
Authors and Affiliations
Contributions
C.C.E. researched data for the article. Both authors wrote the article, made substantial contributions to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.S.E received research support from the Bristol-Myers Squibb–Sanofi Partnership and diaDexus, Inc.; royalties from UpToDate for chapters related to cryptogenic stroke and hemicraniectomy; receives compensation for providing consultative services for Biotelemetry (Cardionet), the Bristol-Myers Squibb–Pfizer Partnership, Boehringer–Ingelheim, and the Sanofi–Regeneron Partnership; serves as the lead Principal Investigator for a Biogen IDEC study of Tysabri® and stroke; and has given expert legal opinions on behalf of Merck (NuvaRing® and stroke litigation), BMS-Sanofi Pharmaceutical Partnership (clopidogrel and stroke litigation), and Hi-Tech Pharmaceuticals (dimethylamylamine and stroke litigation). C.C.E. declares no competing interests.
Glossary
- Acute phase reactants
-
Serum proteins whose concentrations increase as a result of an acute inflammatory state.
- Arterial dolichoectasia
-
Pathological dilation and elongation of arteries through chronic vessel wall remodelling.
- Lacunar strokes
-
Occlusions of a deep penetrating artery leading to infarction of a relatively small cerebral territory.
- Mendelian randomization
-
The random assortment of genetic alleles in a person or a population that can be used as a method to study the risk of having that allele.
Rights and permissions
About this article
Cite this article
Esenwa, C., Elkind, M. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol 12, 594–604 (2016). https://doi.org/10.1038/nrneurol.2016.125
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2016.125
This article is cited by
-
Association between dietary inflammatory index and Stroke in the US population: evidence from NHANES 1999–2018
BMC Public Health (2024)
-
Causal Association of Cytokines and Growth Factors with Stroke and Its Subtypes: a Mendelian Randomization Study
Molecular Neurobiology (2024)
-
Association between clonal hematopoiesis-related gene mutations and unfavorable functional outcome in patients with large-artery atherosclerotic stroke
European Journal of Medical Research (2023)
-
Pathogenesis-adaptive polydopamine nanosystem for sequential therapy of ischemic stroke
Nature Communications (2023)
-
The COP9 signalosome reduces neuroinflammation and attenuates ischemic neuronal stress in organotypic brain slice culture model
Cellular and Molecular Life Sciences (2023)