Abstract
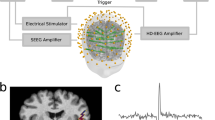
EEG source imaging (ESI) is a model-based imaging technique that integrates temporal and spatial components of EEG to identify the generating source of electrical potentials recorded on the scalp. Recent advances in computer technologies have made the analysis of ESI data less time-consuming, and have rekindled interest in this technique as a clinical diagnostic tool. On the basis of the available body of evidence, ESI seems to be a promising tool for epilepsy evaluation; however, the precise clinical value of ESI in presurgical evaluation of epilepsy and in localization of eloquent cortex remains to be investigated. In this Review, we describe two fundamental issues in ESI; namely, the forward and inverse problems, and their solutions. The clinical application of ESI in surgical planning for patients with medically refractory focal epilepsy, and its use in source reconstruction together with invasive recordings, is also discussed. As ESI can be used to map evoked responses, we discuss the clinical utility of this technique in cortical mapping—an essential process when planning resective surgery for brain regions that are in close proximity to eloquent cortex.
Key Points
-
EEG source imaging (ESI) is a model-based imaging technique that integrates temporal and spatial components of EEG to identify the source of scalp-recorded potentials
-
The choice of forward and inverse solutions can crucially influence the outcome of source localization using ESI
-
A realistic head model using an individual's MRI offers the best forward solution
-
A high total number of electrodes or concentration of electrodes over the region of interest can improve the accuracy of ESI
-
Attention to technical recording details, including co-registration of electrode positions on MRI and modelling of the initial phase of epileptic spikes, is crucial for accurate source localization using ESI
-
On the basis of current evidence, ESI is a promising tool for epilepsy evaluation, but further studies in large epilepsy cohorts are needed to demonstrate its clinical value
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Berger, H. On the human electroencephalogram [German]. Arch. Psychiat. Nervenkr. 87, 527–570 (1929).
Jayakar, P., Duchowny, M., Resnick, T. J. & Alvarez, L. A. Localization of seizure foci: pitfalls and caveats. J. Clin. Neurophysiol. 8, 414–431 (1991).
Helmholtz, H. Ueber einige Gesetze der Vertheilung elektrischer Ströme in körperlichen Leitern mit Anwendung auf die thierisch-elektrischen Versuche [German]. Annalen der Physik und Chemie 165, 211–233 (1853).
Michel, C. M. & Murray, M. M. Towards the utilization of EEG as a brain imaging tool. Neuroimage 61, 371–385 (2012).
van Oosterom, A. History and evolution of methods for solving the inverse problem. J. Clin. Neurophysiol. 8, 371–380 (1991).
Hallez, H. et al. Review on solving the forward problem in EEG source analysis. J. Neuroeng. Rehabil. 4, 46 (2007).
Grech, R. et al. Review on solving the inverse problem in EEG source analysis. J. Neuroeng. Rehabil. 5, 25 (2008).
Pascual-Marqui, R. D., Sekihara, K., Brandeis, D. & Michel, C. M. in Electrical Neuroimaging (eds Michel, C. M. et al.) 49–77 (Cambridge University Press, New York, 2009).
Fuchs, M., Kastner, J., Wagner, M., Hawes, S. & Ebersole, J. S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 113, 702–712 (2002).
Whittingstall, K., Stroink, G., Gates, L., Connolly, J. F. & Finley, A. Effects of dipole position, orientation and noise on the accuracy of EEG source localization. Biomed. Eng. Online 2, 14 (2003).
Michel, C. M. et al. EEG source imaging. Clin. Neurophysiol. 115, 2195–2222 (2004).
Plummer, C., Litewka, L., Farish, S., Harvey, A. S. & Cook, M. J. Clinical utility of current-generation dipole modelling of scalp EEG. Clin. Neurophysiol. 118, 2344–2361 (2007).
Barkley, G. L. & Baumgartner, C. MEG and EEG in epilepsy. J. Clin. Neurophysiol. 20, 163–178 (2003).
He, B. et al. Electric dipole tracing in the brain by means of the boundary element method and its accuracy. IEEE Trans. Biomed. Eng. 34, 406–414 (1987).
Hamalainen, M. S. & Sarvas, J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans. Biomed. Eng. 36, 165–171 (1989).
Miller, C. E. & Henriquez, C. S. Finite element analysis of bioelectric phenomena. Crit. Rev. Biomed. Eng. 18, 207–233 (1990).
Lemieux, L., McBride, A. & Hand, J. W. Calculation of electrical potentials on the surface of a realistic head model by finite differences. Phys. Med. Biol. 41, 1079–1091 (1996).
Huang, M. X., Mosher, J. C. & Leahy, R. M. A sensor-weighted overlapping-sphere head model and exhaustive head model comparison for MEG. Phys. Med. Biol. 44, 423–440 (1999).
Spinelli, L., Andino, S. G., Lantz, G., Seeck, M. & Michel, C. M. Electromagnetic inverse solutions in anatomically constrained spherical head models. Brain Topogr. 13, 115–125 (2000).
Ermer, J. J., Mosher, J. C., Baillet, S. & Leah, R. M. Rapidly recomputable EEG forward models for realistic head shapes. Phys. Med. Biol. 46, 1265–1281 (2001).
Yitembe, B., Crevecoeur, G., Van Keer, R. & Dupre, L. Reduced conductivity dependence method for increase of dipole localization accuracy in the EEG inverse problem. IEEE Trans. Biomed. Eng. 58, 1430–1440 (2011).
Tuch, D. S., Wedeen, V. J., Dale, A. M., George, J. S. & Belliveau, J. W. Conductivity mapping of biological tissue using diffusion MRI. Ann. NY Acad. Sci. 888, 314–316 (1999).
Jain, H., Isaacson, D., Edic, P. M. & Newell, J. C. Electrical impedance tomography of complex conductivity distributions with noncircular boundary. IEEE Trans. Biomed. Eng. 44, 1051–1060 (1997).
Oostendorp, T. F., Delbeke, J. & Stegeman, D. F. The conductivity of the human skull: results of in vivo and in vitro measurements. IEEE Trans. Biomed. Eng. 47, 1487–1492 (2000).
Lai, Y. et al. Estimation of in vivo human brain-to-skull conductivity ratio from simultaneous extra- and intra-cranial electrical potential recordings. Clin. Neurophysiol. 116, 456–465 (2005).
Haueisen, J. et al. The influence of brain tissue anisotropy on human EEG and MEG. Neuroimage 15, 159–166 (2002).
Vatta, F., Meneghini, F., Esposito, F., Mininel, S. & Di Salle, F. Realistic and spherical head modeling for EEG forward problem solution: a comparative cortex-based analysis. Comput. Intell. Neurosci. 972060 (2010).
Ebersole, J. S. & Hawes-Ebersole, S. Clinical application of dipole models in the localization of epileptiform activity. J. Clin. Neurophysiol. 24, 120–129 (2007).
Schneider, M. R. A multistage process for computing virtual dipolar sources of EEG discharges from surface information. IEEE Trans. Biomed. Eng. 19, 1–12 (1972).
Darcey, T. M., Ary, J. P. & Fender, D. H. Spatio-temporal visually evoked scalp potentials in response to partial-field patterned stimulation. Electroencephalogr. Clin. Neurophysiol. 50, 348–355 (1980).
Rose, S. & Ebersole, J. S. Advances in spike localization with EEG dipole modeling. Clin. EEG Neurosci. 40, 281–287 (2009).
Scherg, M. & von Cramon, D. A new interpretation of the generators of BAEP waves I–V: results of a spatio-temporal dipole model. Electroencephalogr. Clin. Neurophysiol. 62, 290–299 (1985).
Achim, A., Richer, F. & Saint-Hilaire, J. M. Methodological considerations for the evaluation of spatio-temporal source models. Electroencephalogr. Clin. Neurophysiol. 79, 227–240 (1991).
Mosher, J. C., Lewis, P. S. & Leahy, R. M. Multiple dipole modeling and localization from spatio-temporal MEG data. IEEE Trans. Biomed. Eng. 39, 541–557 (1992).
Mosher, J. C. & Leahy, R. M. Recursive MUSIC: a framework for EEG and MEG source localization. IEEE Trans. Biomed. Eng. 45, 1342–1354 (1998).
Koles, Z. J., Lind, J. C. & Soong, A. C. Spatio-temporal decomposition of the EEG: a general approach to the isolation and localization of sources. Electroencephalogr. Clin. Neurophysiol. 95, 219–230 (1995).
Kobayashi, K., Akiyama, T., Nakahori, T., Yoshinaga, H. & Gotman, J. Systematic source estimation of spikes by a combination of independent component analysis and RAP-MUSIC. I: principles and simulation study. Clin. Neurophysiol. 113, 713–724 (2002).
Xu, X. L., Xu, B. & He, B. An alternative subspace approach to EEG dipole source localization. Phys. Med. Biol. 49, 327–343 (2004).
Plummer, C., Harvey, A. S. & Cook, M. EEG source localization in focal epilepsy: where are we now? Epilepsia 49, 201–218 (2008).
Pascual-Marqui, R. D., Michel, C. M. & Lehmann, D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int. J. Psychophysiol. 18, 49–65 (1994).
Ebersole, J. S. EEG source modeling. The first word. J. Clin. Neurophysiol. 16, 201–203 (1999).
Scherg, M., Bast, T. & Berg, P. Multiple source analysis of interictal spikes: goals, requirements, and clinical value. J. Clin. Neurophysiol. 16, 214–224 (1999).
Mosher, J. C., Baillet, S. & Leahy, R. M. EEG source localization and imaging using multiple signal classification approaches. J. Clin. Neurophysiol. 16, 225–238 (1999).
Fuchs, M., Wagner, M., Kohler, T. & Wischmann, H. A. Linear and nonlinear current density reconstructions. J. Clin. Neurophysiol. 16, 267–295 (1999).
Michel, C. M. et al. Spatiotemporal EEG analysis and distributed source estimation in presurgical epilepsy evaluation. J. Clin. Neurophysiol. 16, 239–266 (1999).
Ebersole, J. S. EEG source modeling. The last word. J. Clin. Neurophysiol. 16, 297–302 (1999).
Plummer, C., Wagner, M., Fuchs, M., Harvey, A. S. & Cook, M. J. Dipole versus distributed EEG source localization for single versus averaged spikes in focal epilepsy. J. Clin. Neurophysiol. 27, 141–162 (2010).
Baillet, S., Friston, K. & Oostenveld, R. Academic software applications for electromagnetic brain mapping using MEG and EEG. Comput. Intell. Neurosci. 2011, 972050 (2011).
Litvak, V. et al. EEG and MEG data analysis in SPM8. Comput. Intell. Neurosci. 852961 (2011).
Vanrumste, B. et al. Dipole location errors in electroencephalogram source analysis due to volume conductor model errors. Med. Biol. Eng. Comput. 38, 528–534 (2000).
Lantz, G., Grave de Peralta, R., Spinelli, L., Seeck, M. & Michel, C. M. Epileptic source localization with high density EEG: how many electrodes are needed? Clin. Neurophysiol. 114, 63–69 (2003).
Spitzer, A. R., Cohen, L. G., Fabrikant, J. & Hallett, M. A method for determining optimal interelectrode spacing for cerebral topographic mapping. Electroencephalogr. Clin. Neurophysiol. 72, 355–361 (1989).
Srinivasan, R., Nunez, P. L., Tucker, D. M., Silberstein, R. B. & Cadusch, P. J. Spatial sampling and filtering of EEG with spline laplacians to estimate cortical potentials. Brain Topogr. 8, 355–366 (1996).
Tong, S. & Thakor, N. V. Quantitative EEG Analysis Methods and Clinical Applications (Artech House, Boston, 2009).
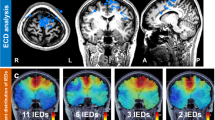
Ding, L. et al. EEG source imaging: correlating source locations and extents with electrocorticography and surgical resections in epilepsy patients. J. Clin. Neurophysiol. 24, 130–136 (2007).
Oliva, M. et al. EEG dipole source localization of interictal spikes in non-lesional TLE with and without hippocampal sclerosis. Epilepsy Res. 92, 183–190 (2010).
Brodbeck, V. et al. Electrical source imaging for presurgical focus localization in epilepsy patients with normal MRI. Epilepsia 51, 583–591 (2010).
Plummer, C. et al. Clinical utility of distributed source modelling of interictal scalp EEG in focal epilepsy. Clin. Neurophysiol. 121, 1726–1739 (2010).
Coutin-Churchman, P. E. et al. Quantification and localization of EEG interictal spike activity in patients with surgically removed epileptogenic foci. Clin. Neurophysiol. 123, 471–485 (2012).
Benar, C. G. & Gotman, J. Non-uniform spatial sampling in EEG source analysis. In Proc. 23rd Annual International Conference of the IEEE 1, 903–905 (2001).
Koessler, L. et al. Spatial localization of EEG electrodes. Neurophysiol. Clin. 37, 97–102 (2007).
Engels, L., De Tiege, X., Op de Beeck, M. & Warzee, N. Factors influencing the spatial precision of electromagnetic tracking systems used for MEG/EEG source imaging. Neurophysiol. Clin. 40, 19–25 (2010).
Gencer, N. G. & Acar, C. E. Sensitivity of EEG and MEG measurements to tissue conductivity. Phys. Med. Biol. 49, 701–717 (2004).
van den Broek, S. P., Reinders, F., Donderwinkel, M. & Peters, M. J. Volume conduction effects in EEG and MEG. Electroencephalogr. Clin. Neurophysiol. 106, 522–534 (1998).
Chitoku, S. et al. Characteristics of dipoles in clustered individual spikes and averaged spikes. Brain Dev. 25, 14–21 (2003).
Fuchs, M., Wagner, M. & Kastner, J. Confidence limits of dipole source reconstruction results. Clin. Neurophysiol. 115, 1442–1451 (2004).
Alarcon, G. et al. Intracerebral propagation of interictal activity in partial epilepsy: implications for source localisation. J. Neurol. Neurosurg. Psychiatry 57, 435–449 (1994).
Wennberg, R., Valiante, T. & Cheyne, D. EEG and MEG in mesial temporal lobe epilepsy: where do the spikes really come from? Clin. Neurophysiol. 122, 1295–1313 (2011).
Rosenow, F. & Lüders, H. Presurgical evaluation of epilepsy. Brain 124, 1683–1700 (2001).
Huppertz, H. J. et al. Cortical current density reconstruction of interictal epileptiform activity in temporal lobe epilepsy. Clin. Neurophysiol. 112, 1761–1772 (2001).
Gavaret, M., Badier, J. M., Marquis, P., Bartolomei, F. & Chauvel, P. Electric source imaging in temporal lobe epilepsy. J. Clin. Neurophysiol. 21, 267–282 (2004).
Gavaret, M. et al. Electric source imaging in frontal lobe epilepsy. J. Clin. Neurophysiol. 23, 358–370 (2006).
Gavaret, M. et al. Source localization of scalp-EEG interictal spikes in posterior cortex epilepsies investigated by HR-EEG and SEEG. Epilepsia 50, 276–289 (2009).
Lantz, G. et al. Propagation of interictal epileptiform activity can lead to erroneous source localizations: a 128-channel EEG mapping study. J. Clin. Neurophysiol. 20, 311–319 (2003).
Michel, C. M. et al. 128-channel EEG source imaging in epilepsy: clinical yield and localization precision. J. Clin. Neurophysiol. 21, 71–83 (2004).
Sperli, F. et al. EEG source imaging in pediatric epilepsy surgery: a new perspective in presurgical workup. Epilepsia 47, 981–990 (2006).
Brodbeck, V. et al. Electroencephalographic source imaging: a prospective study of 152 operated epileptic patients. Brain 134, 2887–2897 (2011).
Mirkovic, N., Adjouadi, M., Yaylali, I. & Jayakar, P. 3-d source localization of epileptic foci integrating EEG and MRI data. Brain Topogr. 16, 111–119 (2003).
Nayak, D. et al. Characteristics of scalp electrical fields associated with deep medial temporal epileptiform discharges. Clin. Neurophysiol. 115, 1423–1435 (2004).
Meckes-Ferber, S., Roten, A., Kilpatrick, C. & O'Brien, T. J. EEG dipole source localisation of interictal spikes acquired during routine clinical video-EEG monitoring. Clin. Neurophysiol. 115, 2738–2743 (2004).
Zumsteg, D., Friedman, A., Wennberg, R. A. & Wieser, H. G. Source localization of mesial temporal interictal epileptiform discharges: correlation with intracranial foramen ovale electrode recordings. Clin. Neurophysiol. 116, 2810–2818 (2005).
Zumsteg, D., Friedman, A., Wieser, H. G. & Wennberg, R. A. Propagation of interictal discharges in temporal lobe epilepsy: correlation of spatiotemporal mapping with intracranial foramen ovale electrode recordings. Clin. Neurophysiol. 117, 2615–2626 (2006).
Merlet, I. & Gotman, J. Dipole modeling of scalp electroencephalogram epileptic discharges: correlation with intracerebral fields. Clin. Neurophysiol. 112, 414–430 (2001).
Boon, P. et al. Ictal source localization in presurgical patients with refractory epilepsy. J. Clin. Neurophysiol. 19, 461–468 (2002).
Tao, J. X., Baldwin, M., Ray, A., Hawes-Ebersole, S. & Ebersole, J. S. The impact of cerebral source area and synchrony on recording scalp electroencephalography ictal patterns. Epilepsia 48, 2167–2176 (2007).
Assaf, B. A. & Ebersole, J. S. Continuous source imaging of scalp ictal rhythms in temporal lobe epilepsy. Epilepsia 38, 1114–1123 (1997).
Koessler, L. et al. Source localization of ictal epileptic activity investigated by high resolution EEG and validated by SEEG. Neuroimage 51, 642–653 (2010).
Assaf, B. A. & Ebersole, J. S. Visual and quantitative ictal EEG predictors of outcome after temporal lobectomy. Epilepsia 40, 52–61 (1999).
Beniczky, S. et al. Source analysis of epileptic discharges using multiple signal classification analysis. Neuroreport 17, 1283–1287 (2006).
Jung, K. Y. et al. Spatiotemporospectral characteristics of scalp ictal EEG in mesial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 1287, 206–219 (2009).
Holmes, M. D. et al. Comparing noninvasive dense array and intracranial electroencephalography for localization of seizures. Neurosurgery 66, 354–362 (2010).
Lantz, G. et al. Frequency domain EEG source localization of ictal epileptiform activity in patients with partial complex epilepsy of temporal lobe origin. Clin. Neurophysiol. 110, 176–184 (1999).
Worrell, G. A. et al. Localization of the epileptic focus by low-resolution electromagnetic tomography in patients with a lesion demonstrated by MRI. Brain Topogr. 12, 273–282 (2000).
Lantz, G. et al. Space-oriented segmentation and 3-dimensional source reconstruction of ictal EEG patterns. Clin. Neurophysiol. 112, 688–697 (2001).
Yang, L., Wilke, C., Brinkmann, B., Worrell, G. A. & He, B. Dynamic imaging of ictal oscillations using non-invasive high-resolution EEG. Neuroimage 56, 1908–1917 (2011).
Lu, Y., Yang, L., Worrell, G. A. & He, B. Seizure source imaging by means of FINE spatio-temporal dipole localization and directed transfer function in partial epilepsy patients. Clin. Neurophysiol. 123, 1275–1283 (2012).
Huppertz, H. J. et al. Localization of interictal delta and epileptiform EEG activity associated with focal epileptogenic brain lesions. Neuroimage 13, 15–28 (2001).
Vanrumste, B., Jones, R. D., Bones, P. J. & Carroll, G. J. Slow-wave activity arising from the same area as epileptiform activity in the EEG of paediatric patients with focal epilepsy. Clin. Neurophysiol. 116, 9–17 (2005).
Alper, K. et al. Localizing epileptogenic regions in partial epilepsy using three-dimensional statistical parametric maps of background EEG source spectra. Neuroimage 39, 1257–1265 (2008).
Fuchs, M., Wagner, M. & Kastner, J. Development of volume conductor and source models to localize epileptic foci. J. Clin. Neurophysiol. 24, 101–119 (2007).
Zhang, Y., van Drongelen, W., Kohrman, M. & He, B. Three-dimensional brain current source reconstruction from intra-cranial ECoG recordings. Neuroimage 42, 683–695 (2008).
Dumpelmann, M., Fell, J., Wellmer, J., Urbach, H. & Elger, C. E. 3D source localization derived from subdural strip and grid electrodes: a simulation study. Clin. Neurophysiol. 120, 1061–1069 (2009).
Wilke, C., van Drongelen, W., Kohrman, M. & He, B. Identification of epileptogenic foci from causal analysis of ECoG interictal spike activity. Clin. Neurophysiol. 120, 1449–1456 (2009).
Kim, J. S. et al. Localization and propagation analysis of ictal source rhythm by electrocorticography. Neuroimage 52, 1279–1288 (2010).
Wilke, C., van Drongelen, W., Kohrman, M. & He, B. Neocortical seizure foci localization by means of a directed transfer function method. Epilepsia 51, 564–572 (2010).
van Mierlo, P. et al. Accurate epileptogenic focus localization through time-variant functional connectivity analysis of intracranial electroencephalographic signals. Neuroimage 56, 1122–1133 (2011).
Sutherling, W. W. et al. The magnetic and electric fields agree with intracranial localizations of somatosensory cortex. Neurology 38, 1705–1714 (1988).
Buchner, H. et al. Source analysis of median nerve and finger stimulated somatosensory evoked potentials: multichannel simultaneous recording of electric and magnetic fields combined with 3D-MR tomography. Brain Topogr. 6, 299–310 (1994).
Bast, T. et al. Combined EEG and MEG analysis of early somatosensory evoked activity in children and adolescents with focal epilepsies. Clin. Neurophysiol. 118, 1721–1735 (2007).
Bai, X., Towle, V. L., van Drongelen, W. & He, B. Cortical potential imaging of somatosensory evoked potentials by means of the boundary element method in pediatric epilepsy patients. Brain Topogr. 23, 333–343 (2011).
Rampp, S. & Stefan, H. On the opposition of EEG and MEG. Clin. Neurophysiol. 118, 1658–1659 (2007).
Acknowledgements
K. Kaiboriboon and M. Hamaneh are supported by the Epilepsy Foundation. M. Hamaneh is also supported by the Coulter Foundation.
Author information
Authors and Affiliations
Contributions
K. Kaiboriboon researched data for the article. K. Kaiboriboon and S. D. Lhatoo provided substantial contributions to discussion of content and wrote the article. K. Kaiboriboon, H. O. Lüders, M. Hamaneh, J. Turnbull and S. D. Lhatoo contributed equally to review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
EEG source imaging software packages (DOC 57 kb)
Supplementary Table 2
Clinical studies of interictal source analysis (DOC 82 kb)
Supplementary Table 3
Clinical studies of ictal source analysis (DOC 52 kb)
Rights and permissions
About this article
Cite this article
Kaiboriboon, K., Lüders, H., Hamaneh, M. et al. EEG source imaging in epilepsy—practicalities and pitfalls. Nat Rev Neurol 8, 498–507 (2012). https://doi.org/10.1038/nrneurol.2012.150
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2012.150
This article is cited by
-
Big Field of View MRI T1w and FLAIR Template - NMRI225
Scientific Data (2023)
-
Normative brain mapping using scalp EEG and potential clinical application
Scientific Reports (2023)
-
EEG/MEG-Quellenrekonstruktion bei nichtläsioneller Epilepsie
Clinical Epileptology (2023)
-
An experimental validation of partial discharge localization using electromagnetic time reversal
Scientific Reports (2021)
-
Diagnosis and surgical treatment of non-lesional temporal lobe epilepsy with unilateral amygdala enlargement
Neurological Sciences (2021)