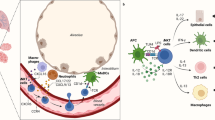
Abstract
In several mouse models, natural killer T cells have recently been found to be required for the development of airway hyper-reactivity, a cardinal feature of asthma. Moreover, in patients with chronic asthma, natural killer T cells with a T-helper-2-like phenotype (that is, that express CD4 and produce T helper 2 cytokines) are present in the lungs in large numbers. In this Opinion article, we suggest that natural killer T cells, which express a restricted T-cell receptor and respond to glycolipids rather than protein antigens, have a previously unsuspected but crucial role, distinct from that of T helper 2 cells, in the pathogenesis of asthma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kon, O. M. et al. The effects of an anti-CD4 monoclonal antibody, keliximab, on peripheral blood CD4+ T-cells in asthma. Eur. Respir. J. 18, 45–52 (2001).
Gavett, S. H., Chen, X., Finkelman, F. & Wills-Karp, M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am. J. Respir. Cell Mol. Biol. 10, 587–593 (1994).
Azzawi, M. et al. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am. Rev. Respir. Dis. 142, 1407–1413 (1990).
Robinson, D. S. et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 326, 298–304 (1992).
Hansen, G., Berry, G., DeKruyff, R. H. & Umetsu, D. T. Allergen-specific TH1 cells fail to counterbalance TH2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 103, 175–183 (1999).
Randolph, D. A., Carruthers, C. J., Szabo, S. J., Murphy, K. M. & Chaplin, D. D. Modulation of airway inflammation by passive transfer of allergen-specific TH1 and TH2 cells in a mouse model of asthma. J. Immunol. 162, 2375–2383 (1999).
Cohn, L., Tepper, J. S. & Bottomly, K. IL-4-independent induction of airway hyperresponsiveness by TH2, but not TH1, cells. J. Immunol. 161, 3813–3816 (1998).
Corry, D. B. et al. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol. Med. 4, 344–355 (1998).
Godfrey, D. I. & Kronenberg, M. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114, 1379–1388 (2004).
Taniguchi, M., Harada, M., Kojo, S., Nakayama, T. & Wakao, H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21, 483–513 (2003).
Exley, M. A. & Koziel, M. J. To be or not to be NKT: natural killer T cells in the liver. Hepatology 40, 1033–1040 (2004).
Seino, K. & Taniguchi, M. Functionally distinct NKT cell subsets and subtypes. J. Exp. Med. 202, 1623–1626 (2005).
Fuss, I. J. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical TH2 response in ulcerative colitis. J. Clin. Invest. 113, 1490–1497 (2004).
Terabe, M. et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R–STAT6 pathway. Nature Immunol. 1, 515–520 (2000).
Akbari, O. et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nature Med. 9, 582–588 (2003).
Lisbonne, M. et al. Invariant Vα14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 171, 1637–1641 (2003).
Brigl, M. & Brenner, M. B. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22, 817–890 (2004).
Kinjo, Y. et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434, 520–525 (2005).
Zhou, D. P. et al. Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 (2004).
Kronenberg, M. & Rudensky, A. Regulation of immunity by self-reactive T cells. Nature 435, 598–604 (2005).
Wilson, S. B. et al. Extreme TH1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature 391, 177–181 (1998).
Kinjo, Y. et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nature Immunol. 7, 978–986 (2006).
Crowe, N. Y. et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 202, 1279–1288 (2005).
Lowsky, R. et al. Protective conditioning for acute graft-versus-host disease. N. Engl. J. Med. 353, 1321–1331 (2005).
Zeng, D. et al. Activation of natural killer T cells in NZB/W mice induces TH1-type immune responses exacerbating lupus. J. Clin. Invest. 112, 1211–1222 (2003).
Bilenki, L., Yang, J., Fan, Y., Wang, S. & Yang, X. Natural killer T cells contribute to airway eosinophilic inflammation induced by ragweed through enhanced IL-4 and eotaxin production. Eur. J. Immunol. 34, 345–354 (2004).
Cui, J. et al. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Vα14 natural killer T cells. J. Exp. Med. 190, 783–792 (1999).
Smiley, S. T., Kaplan, M. H. & Grusby, M. J. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science 275, 977–979 (1997).
Yoshimoto, T., Bendelac, A., Hu-Li, J. & Paul, W. E. Defective IgE production by SJL mice is linked to the absence of CD4+, NK1.1+ T cells that promptly produce interleukin 4. Proc. Natl Acad. Sci. USA 92, 11931–11934 (1995).
Kim, J. O. et al. Asthma is induced by intranasal coadministration of allergen and natural killer T-cell ligand in a mouse model. J. Allergy Clin. Immunol. 114, 1332–1338 (2004).
Meyer, E. H. et al. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc. Natl Acad. Sci. USA 103, 2782–2787 (2006).
Bendelac, A., Hunziker, R. D. & Lantz, O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J. Exp. Med. 184, 1285–1293 (1996).
Yoshimoto, T., Bendelac, A., Watson, C., Hu-Li, J. & Paul, W. E. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science 270, 1845–1847 (1995).
Galli, G. et al. Innate immune responses support adaptive immunity: NKT cells induce B cell activation. Vaccine 21 (Suppl. 2), 48–54 (2003).
Korsgren, M. et al. Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice. J. Exp. Med. 189, 553–562 (1999).
Brown, D. et al. β2-Microglobulin-dependent NK1.1+ T cells are not essential for T helper cell 2 immune responses. J. Exp. Med. 184, 1295–1304 (1996).
Zhang, Y., Rogers, K. H. & Lewis, D. B. β2-Microglobulin-dependent T cells are dispensable for allergen-induced T helper 2 responses. J. Exp. Med. 184, 1507–1512 (1996).
Amano, M. et al. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: β2-microglobulin-dependent and independent forms. J. Immunol. 161, 1710–1717 (1998).
Maeda, M., Shadeo, A., MacFadyen, A. & Takei, F. CD1d-independent NKT cells in β2-microglobulin-deficient mice have hybrid phenotype and function of NK and T cells. J. Immunol. 172, 6115–6122 (2004).
Kim, H. S. et al. Biochemical characterization of CD1d expression in the absence of β2-microglobulin. J. Biol. Chem. 274, 9289–9295 (1999).
Townsend, M. J. et al. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity 20, 477–494 (2004).
Finotto, S. et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science 295, 336–338 (2002).
Benlagha, K., Kyin, T., Beavis, A., Teyton, L. & Bendelac, A. A thymic precursor to the NK T cell lineage. Science 296, 553–555 (2002).
Akbari, O. et al. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N. Engl. J. Med. 354, 1117–1129 (2006).
Ikegami, Y., Yokoyama, A., Haruta, Y., Hiyama, K. & Kohno, N. Circulating natural killer T cells in patients with asthma. J. Asthma 41, 877–882 (2004).
Gumperz, J. E., Miyake, S., Yamamura, T. & Brenner, M. B. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 195, 625–636 (2002).
Lee, P. T., Benlagha, K., Teyton, L. & Bendelac, A. Distinct functional lineages of human Vα24 natural killer T cells. J. Exp. Med. 195, 637–641 (2002).
Johnston, B., Kim, C., Soler, D., Emoto, M. & Butcher, E. Differential chemokine responses and homing patterns of murine TCRαβ NKT cell subsets. J. Immunol. 171, 2960–2969 (2003).
Mattner, J. et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Agea, E. et al. Human CD1-restricted T cell recognition of lipids from pollens. J. Exp. Med. 202, 295–308 (2005).
Adcock, I. M. & Ito, K. Steroid resistance in asthma: a major problem requiring novel solutions or a non-issue? Curr. Opin. Pharmacol. 4, 257–262 (2004).
Ito, K., Chung, K. F. & Adcock, I. M. Update on glucocorticoid action and resistance. J. Allergy Clin. Immunol. 117, 522–543 (2006).
Milner, J. D. et al. Differential responses of invariant Vα24JαQ T cells and MHC class II-restricted CD4+ T cells to dexamethasone. J. Immunol. 163, 2522–2529 (1999).
Tamada, K., Harada, M., Abe, K., Li, T. & Nomoto, K. IL-4-producing NK1.1+ T cells are resistant to glucocorticoid-induced apoptosis: implications for the TH1/TH2 balance. J. Immunol. 161, 1239–1247 (1998).
Hachem, P. et al. α-Galactosylceramide-induced iNKT cells suppress experimental allergic asthma in sensitized mice: role of IFN-γ. Eur. J. Immunol. 35, 2793–2802 (2005).
Matsuda, H. et al. α-Galactosylceramide, a ligand of natural killer T cells, inhibits allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 33, 22–31 (2005).
Morishima, Y. et al. Suppression of eosinophilic airway inflammation by treatment with α-galactosylceramide. Eur. J. Immunol. 35, 2803–2814 (2005).
Parekh, V. V. et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J. Clin. Invest. 115, 2572–2583 (2005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dale Umetsu is a consultant for Innate Immune Inc. (United States).
Related links
Rights and permissions
About this article
Cite this article
Umetsu, D., DeKruyff, R. A role for natural killer T cells in asthma. Nat Rev Immunol 6, 953–958 (2006). https://doi.org/10.1038/nri1968
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1968
This article is cited by
-
Natural killer T cells and the regulation of asthma
Mucosal Immunology (2009)