Key Points
-
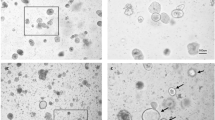
Human 'mini-guts' generated from crypt-based or induced pluripotent stem (iPS) cells functionally recapitulate normal intestinal transport physiology and model pathophysiologic changes following interactions with enteric pathogens
-
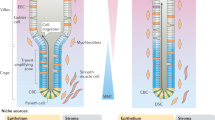
iPS-cell-derived intestinal organoids can be used to model intestinal development and engrafted in vivo to differentiate the epithelium along a crypt–villus axis supported by subepithelial and smooth muscle layers
-
3D enteroids and colonoids derived from intestinal stem cells can be used to measure ion, nutrient and water absorption or secretion as part of normal intestinal transport function
-
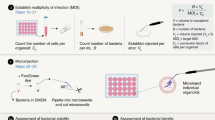
Both human intestinal organoids and enteroids or colonoids can be used as models to study enteric bacterial and viral pathogenesis
-
Enteroids and colonoids can be grown on 2D permeable supports to enable apical access to study host–pathogen interactions as well as drug absorption and/or metabolism
-
Stem cell or iPS-cell-derived gastrointestinal organoids from the stomach, pancreas and liver are also being developed as models to study development, infection and regenerative medicine
Abstract
The development of indefinitely propagating human 'mini-guts' has led to a rapid advance in gastrointestinal research related to transport physiology, developmental biology, pharmacology, and pathophysiology. These mini-guts, also called enteroids or colonoids, are derived from LGR5+ intestinal stem cells isolated from the small intestine or colon. Addition of WNT3A and other growth factors promotes stemness and results in viable, physiologically functional human intestinal or colonic cultures that develop a crypt–villus axis and can be differentiated into all intestinal epithelial cell types. The success of research using human enteroids has highlighted the limitations of using animals or in vitro, cancer-derived cell lines to model transport physiology and pathophysiology. For example, curative or preventive therapies for acute enteric infections have been limited, mostly due to the lack of a physiological human intestinal model. However, the human enteroid model enables specific functional studies of secretion and absorption in each intestinal segment as well as observations of the earliest molecular events that occur during enteric infections. This Review describes studies characterizing these human mini-guts as a physiological model to investigate intestinal transport and host–pathogen interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Seidler, U. E. Gastrointestinal HCO3− transport and epithelial protection in the gut: new techniques, transport pathways and regulatory pathways. Curr. Opin. Pharmacol. 13, 900–908 (2013).
Canny, G. O. & McCormick, B. A. Bacteria in the intestine, helpful residents or enemies from within? Infect. Immun. 76, 3360–33738 (2008).
Heath, J. P. Epithelial cell migration in the intestine. Cell Biol. Int. 20, 139–146 (1996).
Cheng, H. & Bjerknes, M. Whole population cell kinetics and postnatal development of the mouse intestinal epithelium. Anat. Rec. 211, 420–426 (1985).
Barker, N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 15, 19–33 (2013).
Artursson, P. Epithelial transport of drugs in cell culture. I: a model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J. Pharm. Sci. 79, 476–482 (1990).
Middendorp, S. et al. Adult stem cells in the small intestine are intrinsically programmed with their location-specific function. Stem Cells 32, 1083–1091 (2014).
Foulke-Abel, J. et al. Human enteroids as a model of upper small intestinal ion transport physiology and pathophysiology. Gastroenterology 150, 638–649 (2015).
In, J. et al. Enterohemorrhagic Escherichia coli reduces mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell. Mol. Gastroenterol. Hepatol. 2, 48–62.e43 (2016).
McCracken, K. W., Howell, J. C., Wells, J. M. & Spence, J. R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 6, 1920–1928 (2011).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762–1772 (2011).
Casburn-Jones, A. C. Management of infectious diarrhoea. Gut 53, 296–305 (2004).
Thiagarajah, J. R., Donowitz, M. & Verkman, A. S. Secretory diarrhoea: mechanisms and emerging therapies. Nat. Rev. Gastroenterol. Hepatol. 12, 446–457 (2015).
Hilgers, A. R., Conradi, R. A. & Burton, P. S. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm. Res. 7, 902–910 (1990).
Nataro, J. P., Hicks, S., Phillips, A. D., Vial, P. A. & Sears, C. L. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect. Immun. 64, 4761–4768 (1996).
Huet, C., Sahuquillo-Merino, C., Coudrier, E. & Louvard, D. Absorptive and mucus-secreting subclones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J. Cell Biol. 105, 345–357 (1987).
Larregieu, C. A. & Benet, L. Z. Drug discovery and regulatory considerations for improving in silico and in vitro predictions that use Caco-2 as a surrogate for human intestinal permeability measurements. AAPS J. 15, 483–497 (2013).
Sun, D. et al. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharm. Res. 19, 1400–1416 (2002).
Awortwe, C., Fasinu, P. S. & Rosenkranz, B. Application of Caco-2 cell line in herb–drug interaction studies: current approaches and challenges. J. Pharm. Pharm. Sci. 17, 1–19 (2014).
Artursson, P., Palm, K. & Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 46, 27–43 (2001).
Hughes, P., Marshall, D., Reid, Y., Parkes, H. & Gelber, C. The costs of using unauthenticated, over-passaged cell lines: how much more data do we need? BioTechniques 43, 575–586 (2007).
Markert, T. et al. Endogenous expression of type II cGMP-dependent protein kinase mRNA and protein in rat intestine. Implications for cystic fibrosis transmembrane conductance regulator. J. Clin. Invest. 96, 822–830 (1995).
Vaandrager, A. B. et al. Differential role of cyclic GMP-dependent protein kinase II in ion transport in murine small intestine and colon. Gastroenterology 118, 108–114 (2000).
van der Mark, V. A. et al. The lipid flippase heterodimer ATP8B1–CDC50A is essential for surface expression of the apical sodium-dependent bile acid transporter (SLC10A2/ASBT) in intestinal Caco-2 cells. Biochim. Biophys. Acta 1842, 2378–2386 (2014).
Johansson, M. E. V. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008).
Erdem, A. L., Avelino, F., Xicohtencatl-Cortes, J. & Giron, J. A. Host protein binding and adhesive properties of H6 and H7 flagella of attaching and effacing Escherichia coli. J. Bacteriol. 189, 7426–7435 (2007).
Pacheco, A. R. et al. Fucose sensing regulates bacterial intestinal colonization. Nature 492, 113–117 (2012).
Spence, J. R. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011).
Finkbeiner, S. R. et al. Transcriptome-wide analysis reveals hallmarks of human intestine development and maturation in vitro and in vivo. Stem Cell Reports 4, 1140–1155 (2015).
Watson, C. L. et al. An in vivo model of human small intestine using pluripotent stem cells. Nat. Med. 20, 1310–1314 (2014).
Wells, J. M. & Spence, J. R. How to make an intestine. Development 141, 752–760 (2014).
Sinagoga, K. L. & Wells, J. M. Generating human intestinal tissues from pluripotent stem cells to study development and disease. EMBO J. 34, 1149–1163 (2015).
Stelzner, M. et al. A nomenclature for intestinal in vitro cultures. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1359–G1363 (2012).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2010).
Zachos, N. C. et al. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J. Biol. Chem. 291, 3759–3766 (2015).
Sato, T. & Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194 (2013).
Kovbasnjuk, O. et al. Human enteroids: preclinical models of non-inflammatory diarrhea. Stem Cell Res. Ther. 4 (Suppl. 1), S3 (2013).
Foulke-Abel, J. et al. Human enteroids as an ex-vivo model of host-pathogen interactions in the gastrointestinal tract. Exp. Biol. Med. 239, 1124–1134 (2014).
Fujii, M., Matano, M., Nanki, K. & Sato, T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 10, 1474–1485 (2015).
Drost, J. et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 521, 43–47 (2015).
Date, S. & Sato, T. Mini-gut organoids: reconstitution of stem cell niche. Annu. Rev. Cell Dev. Biol. 31, 269–289 (2015).
de Lau, W. et al. Peyer's patch M cells derived from Lgr5+ stem cells require SpiB and are induced by RankL in cultured “miniguts”. Mol. Cell. Biol. 32, 3639–3647 (2012).
Schwank, G. et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658 (2013).
Matano, M. et al. Modeling colorectal cancer using CRISPR–Cas9-mediated engineering of human intestinal organoids. Nat. Med. 21, 256–262 (2015).
Yui, S. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 18, 618–623 (2012).
Dekkers, J. F. et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 19, 939–945 (2013).
Turnberg, L. A., Bieberdorf, F. A., Morawski, S. G. & Fordtran, J. S. Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J. Clin. Invest. 49, 557–567 (1970).
Turnberg, L. A., Fordtran, J. S., Carter, N. W. & Rector, F. C. Mechanism of bicarbonate absorption and its relationship to sodium transport in the human jejunum. J. Clin. Invest. 49, 548–556 (1970).
Kunzelmann, K. & Mall, M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol. Rev. 82, 245–289 (2002).
Frizzell, R. A., Field, M. & Schultz, S. G. Sodium-coupled chloride transport by epithelial tissues. Am. J. Physiol. Renal Physiol. 236, F1–F8 (1979).
Field, M., Fromm, D., Al-Awqati, Q. & Greenough, W. B. Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J. Clin. Invest. 51, 796–804 (1972).
Field, M., Graf, L. H. Jr, Laird, W. J. & Smith, P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc. Natl Acad. Sci. USA 75, 2800–2804 (1978).
Dharmsathaphorn, K., Mandel, K. G., Masui, H. & McRoberts, J. A. Vasoactive intestinal polypeptide-induced chloride secretion by a colonic epithelial cell line. Direct participation of a basolaterally localized Na+,K+,Cl− cotransport system. J. Clin. Invest. 75, 462–471 (1985).
Mandel, K. G., Dharmsathaphorn, K. & McRoberts, J. A. Characterization of a cyclic AMP-activated Cl− tranport pathway in the apical membrane of a human colonic epithelial cell line. J. Biol. Chem. 261, 704–712 (1986).
Dharmsathaphorn, K. & Pandol, S. J. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J. Clin. Invest. 77, 348–354 (1986).
Musch, M. W., Arvans, D. L., Wu, G. D. & Chang, E. B. Functional coupling of the downregulated in adenoma Cl−/base exchanger DRA and the apical Na+/H+ exchangers NHE2 and NHE3. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G202–G210 (2009).
Mizutani, T. et al. Real-time analysis of P-glycoprotein-mediated drug transport across primary intestinal epithelium three-dimensionally cultured in vitro. Biochem. Biophys. Res. Commun. 419, 238–243 (2012).
Grant, C. N. et al. Human and mouse tissue-engineered small intestine both demonstrate digestive and absorptive function. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G664–G677 (2014).
Saxena, K. et al. Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J. Virol. 90, 43–56 (2016).
Walker, N. M. et al. Cellular chloride and bicarbonate retention alters intracellular pH regulation in Cftr KO crypt epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G70–G80 (2015).
Abba, K., Sinfield, R., Hart, C. A. & Garner, P. Pathogens associated with persistent diarrhoea in children in low and middle income countries: systematic review. BMC Infect. Dis. 9, 88 (2009).
Fletcher, S. M., McLaws, M.-L. & Ellis, J. T. Prevalence of gastrointestinal pathogens in developed and developing countries: systemic review and meta-analysis. J. Public Health Res. 2, 42–53 (2013).
Page, A.-L. et al. Geographic distribution and mortality risk factors during the cholera outbreak in a rural region of Haiti, 2010–2011. PLoS Negl. Trop. Dis. 9, e0003605 (2015).
Jenkins, C. et al. Public health investigation of two outbreaks of Shiga toxin-producing Escherichia coli O157 associated with consumption of watercress. Appl. Environ. Microbiol. 81, 3946–3952 (2015).
Luna-Gierke, R. E. et al. Outbreaks of non-O157 Shiga toxin-producing Escherichia coli infection: USA. Epidemiol. Infect. 142, 2270–2280 (2014).
Navarro-Garcia, F. Escherichia coli O104:H4 pathogenesis: an enteroaggregative E. coli/Shiga toxin-producing E. coli explosive cocktail of high virulence. Microbiol. Spectr. 2, 533–539 (2014).
Warny, M. et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366, 1079–1084 (2005).
Martinez, F. Clostridium difficile outbreaks: prevention and treatment strategies. Risk Manag. Healthc. Policy 5, 55–64 (2012).
Brown, K., Valenta, K., Fisman, D., Simor, A. & Daneman, N. Hospital ward antibiotic prescribing and the risks of Clostridium difficile Infection. JAMA Intern. Med. 175, 626–633 (2015).
Davies, J. & Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 74, 417–433 (2010).
Garner, C. D. et al. Perturbation of the small intestine microbial ecology by streptomycin alters pathology in a Salmonella enterica Serovar Typhimurium murine model of infection. Infect. Immun. 77, 2691–2702 (2009).
Hodges, K. & Gill, R. Infectious diarrhea cellular and molecular mechanisms. Gut Microbes 1, 4–21 (2010).
Willing, B. P., Russell, S. L. & Finlay, B. B. Shifting the balance: antibiotic effects on host–microbiota mutualism. Nat. Rev. Microbiol. 9, 233–243 (2011).
Jiminez, J. A., Uwiera, T. C., Douglas Inglis, G. & Uwiera, R. R. E. Animal models to study acute and chronic intestinal inflammation in mammals. Gut Pathog. 7, 29 (2015).
Engevik, M. A. et al. Human Clostridium difficile infection: altered mucus production and composition. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G510–G524 (2014).
Leslie, J. L. et al. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect. Immun. 83, 138–145 (2015).
Engevik, M. A. et al. Human Clostridium difficile infection: inhibition of NHE3 and microbiota profile. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G497–G509 (2014).
Hayashi, H. et al. Inhibition and redistribution of NHE3, the apical Na+/H+ exchanger, by Clostridium difficile toxin B. J. Gen. Physiol. 123, 491–504 (2004).
Forbester, J. L. et al. Interaction of Salmonella enterica serovar Typhimurium with intestinal organoids derived from human induced pluripotent stem cells. Infect. Immun. 83, 2926–2934 (2015).
Finkbeiner, S. R. et al. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. mBio 3, e00159-12 (2012).
Yin, Y. et al. Modeling rotavirus infection and antiviral therapy using primary intestinal organoids. Antiviral Res. 123, 120–131 (2015).
VanDussen, K. L. et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64, 911–920 (2015).
Ermund, A., Schutte, A., Johansson, M. E. V., Gustafsson, J. K. & Hansson, G. C. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer's patches. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G341–G347 (2013).
Johansson, M. E. V., Sjövall, H. & Hansson, G. C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 352–361 (2013).
Stange, Daniel, E. et al. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155, 357–368 (2013).
Bartfeld, S. et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148, 126–136.e6 (2015).
Schlaermann, P. et al. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut 65, 202–213 (2014).
Barker, N. et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 (2010).
McCracken, K. W. et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404 (2014).
Chen, Y.-J. et al. De novo formation of insulin-producing “neo-β cell islets” from intestinal crypts. Cell Rep. 6, 1046–1058 (2014).
Guye, P. et al. Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6. Nat. Commun. 7, 10243 (2016).
Huch, M. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013).
Huch, M. et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312 (2014).
Park, J.-G. et al. Characteristics of cell lines established from human gastric carcinoma. Cancer Res. 50, 2773–2780 (1990).
Leite, M. & Figueiredo, C. A. Method for short-term culture of human gastric epithelial cells to study the effects of Helicobacter pylori. 921, 61–68 (2012).
Graham, D. Y. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J. Gastroenterol. 20, 5191–204 (2014).
Bertaux-Skeirik, N. et al. CD44 plays a functional role in Helicobacter pylori-induced epithelial cell proliferation. PLOS Pathog. 11, e1004663 (2015).
Takaishi, S. et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 27, 1006–1020 (2009).
Takebe, T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013).
Acknowledgements
The authors' research is supported by NIH grants K01DK106323 (J.G.I.), R01DK026523 (M.D.), R01DK061765 (M.D.), P30DK089502 (M.D.), T32DK007632 (M.D.), UH3TR000503 (M.D.), UH3TR000504 (M.D.), U01DK10316 (M.K.E.), and the Bill and Melinda Gates Foundation: Grand Challenges (M.D., O.K., M.K.E.).
Author information
Authors and Affiliations
Contributions
M.D. and J.G.I. researched data, contributed to discussion of content and writing, and reviewed/edited the manuscript before submission. J.F.-A. researched data for the article and contributed to writing. M.K.E. reviewed/edited the manuscript before submission. N.C.Z. and O.K. researched data for the article and contributed to discussion of content and writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
In, J., Foulke-Abel, J., Estes, M. et al. Human mini-guts: new insights into intestinal physiology and host–pathogen interactions. Nat Rev Gastroenterol Hepatol 13, 633–642 (2016). https://doi.org/10.1038/nrgastro.2016.142
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.142
This article is cited by
-
Blockade of interferon signaling decreases gut barrier integrity and promotes severe West Nile virus disease
Nature Communications (2023)
-
Gut microbiota and cardiac arrhythmia: a pharmacokinetic scope
The Egyptian Heart Journal (2022)
-
Generation of CRISPR–Cas9-mediated genetic knockout human intestinal tissue–derived enteroid lines by lentivirus transduction and single-cell cloning
Nature Protocols (2022)
-
Intestinal organoids in farm animals
Veterinary Research (2021)
-
Porcine small intestinal organoids as a model to explore ETEC–host interactions in the gut
Veterinary Research (2021)