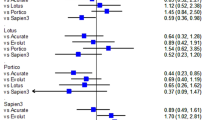
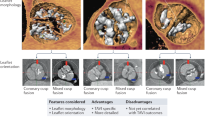
Key Points
-
Transcatheter aortic valve implantation (TAVI) is now a viable therapeutic option for a wide variety of patients, through its numerous vascular access approaches and different valve systems
-
The process of selecting the appropriate TAVI device and procedure for each individual patient requires a comprehensive and multidisciplinary approach
-
Numerous clinical situations require special considerations, including the presence of low coronary ostia, pre-existing mitral prostheses, bicuspid aortic valves, severe left ventricular outflow tract calcification, and degenerative bioprosthetic valve disease
-
As techniques evolve to treat younger patients and lower-risk populations, issues associated with procedural safety, such as risk of neurological events, paravalvular aortic regurgitation, and permanent pacemaker placement, must be addressed with newer-generation devices
Abstract
Transcatheter aortic valve implantation (TAVI) has become a widely accepted strategy for the treatment of aortic stenosis in patients at intermediate, high, or prohibitive surgical risk. After >1 decade of innovation and clinical trial experience, the available technology for TAVI has grown enormously, and now includes a myriad of vascular access approaches and innovative valve designs. As a result, the range of patients who can benefit from these advances continues to grow rapidly. Furthermore, given the improved safety profile and clinical success of current-generation devices in randomized trials, the use of TAVI among even low-risk populations is justified in current trials. With the rapid dissemination and expansion of this technology, operators need to have a comprehensive understanding of how to select the appropriate procedural approach for each individual patient. In this Review, we detail the current evidence for TAVI among different patient populations, discuss the different vascular access approaches currently in use, and explore differences in design features among currently available and investigational valve systems. Furthermore, we provide an overview of important considerations for special patient populations, such as those with existing mitral prostheses, bicuspid aortic stenosis, isolated aortic regurgitation, or severe left ventricular outflow tract calcification.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Freeman, R. V. & Otto, C. M. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 111, 3316–3326 (2005).
Nishimura, R. A. et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J. Am. Coll. Cardiol. 63, e57–e185 (2014).
Ross, J. Jr & Braunwald, E. Aortic stenosis. Circulation 38, 61–67 (1968).
Iung, B. et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur. Heart J. 26, 2714–2720 (2005).
O'Neill, W. W. Predictors of long-term survival after percutaneous aortic valvuloplasty: report of the Mansfield Scientific Balloon Aortic Valvuloplasty Registry. J. Am. Coll. Cardiol. 17, 193–198 (1991).
Kapadia, S. et al. Outcomes of inoperable symptomatic aortic stenosis patients not undergoing aortic valve replacement: insight into the impact of balloon aortic valvuloplasty from the PARTNER trial (Placement of AoRtic TraNscathetER Valve trial). JACC Cardiovasc. Interv. 8, 324–333 (2015).
Cribier, A. et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 106, 3006–3008 (2002).
Leon, M. B. et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363, 1597–1607 (2010).
Kapadia, S. R. et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (partner 1): a randomised controlled trial. Lancet 385, 2485–2491 (2015).
Popma, J. J. et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J. Am. Coll. Cardiol. 63, 1972–1981 (2014).
Smith, C. R. et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 364, 2187–2198 (2011).
Mack, M. J. et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (partner 1): a randomised controlled trial. Lancet 385, 2477–2484 (2015).
Adams, D. H. et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 370, 1790–1798 (2014).
Reardon, M. J. et al. 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 66, 113–121 (2015).
Tamburino, C. et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation 123, 299–308 (2011).
Athappan, G. et al. Predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J. Am. Coll. Cardiol. 61, 1585–1595 (2013).
Kapadia, S. R. et al. Cerebral embolic protection during transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 69, 367–377 (2017).
Giustino, G., Sorrentino, S., Mehran, R., Faggioni, M. & Dangas, G. Cerebral embolic protection during TAVR: a clinical event meta-analysis. J. Am. Coll. Cardiol. 69, 465–466 (2017).
Reynolds, M. R. et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRtic TraNscathetER Valve) trial (Cohort A). J. Am. Coll. Cardiol. 60, 548–558 (2012).
Reynolds, M. R. et al. Cost-effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results of the PARTNER (Placement of Aortic Transcatheter Valves) trial (Cohort A). J. Am. Coll. Cardiol. 60, 2683–2692 (2012).
Piazza, N. et al. 3-center comparison of 1-year mortality outcomes between transcatheter aortic valve implantation and surgical aortic valve replacement on the basis of propensity score matching among intermediate-risk surgical patients. JACC Cardiovasc. Interv. 6, 443–451 (2013).
Latib, A. et al. Transcatheter versus surgical aortic valve replacement in intermediate-surgical-risk patients with aortic stenosis: a propensity score-matched case-control study. Am. Heart J. 164, 910–917 (2012).
Tamburino, C. et al. 1-year outcomes after transfemoral transcatheter or surgical aortic valve replacement: results from the Italian OBSERVANT study. J. Am. Coll. Cardiol. 66, 804–812 (2015).
Schymik, G. et al. A comparison of transcatheter aortic valve implantation and surgical aortic valve replacement in 1,141 patients with severe symptomatic aortic stenosis and less than high risk. Catheter Cardiovasc. Interv. 86, 738–744 (2015).
Muneretto, C. et al. Treating the patients in the 'grey-zone' with aortic valve disease: a comparison among conventional surgery, sutureless valves and transcatheter aortic valve replacement. Interact. Cardiovasc. Thorac. Surg. 20, 90–95 (2015).
Leon, M. B. et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 374, 1609–1620 (2016).
Thourani, V. H. et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 387, 2218–2225 (2016).
Nishimura, R. A. et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J. Am. Coll. Cardiol. http://dx.doi.org/10.1161/CIR.0000000000000503 (2017).
Reardon, M. J. et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 376, 1321–1331 (2017).
Thyregod, H. G. et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers notion randomized clinical trial. J. Am. Coll. Cardiol. 65, 2184–2194 (2015).
Rosato, S. et al. Transcatheter aortic valve implantation compared with surgical aortic valve replacement in low-risk patients. Circ. Cardiovasc. Interv. 9, e003326 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02675114 (2017).
Nombela-Franco, L. et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: a comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J. Am. Coll. Cardiol. 63, 2643–2658 (2014).
Unger, P., Clavel, M. A., Lindman, B. R., Mathieu, P. & Pibarot, P. Pathophysiology and management of multivalvular disease. Nat. Rev. Cardiol. 13, 429–440 (2016).
Danson, E. et al. Assessment, treatment, and prognostic implications of CAD in patients undergoing TAVI. Nat. Rev. Cardiol. 13, 276–285 (2016).
Pasic, M., Unbehaun, A., Buz, S., Drews, T. & Hetzer, R. Annular rupture during transcatheter aortic valve replacement: classification, pathophysiology, diagnostics, treatment approaches, and prevention. JACC Cardiovasc. Interv. 8, 1–9 (2015).
Khatri, P. J. et al. Adverse effects associated with transcatheter aortic valve implantation: a meta-analysis of contemporary studies. Ann. Intern. Med. 158, 35–46 (2013).
Zhao, Z. G., Jilaihawi, H., Feng, Y. & Chen, M. Transcatheter aortic valve implantation in bicuspid anatomy. Nat. Rev. Cardiol. 12, 123–128 (2015).
Agarwal, S. et al. Transcatheter aortic valve replacement: current perspectives and future implications. Heart 101, 169–177 (2015).
Gilard, M. et al. Late outcomes of transcatheter aortic valve replacement in high-risk patients: the France-2 registry. J. Am. Coll. Cardiol. 68, 1637–1647 (2016).
Mack, M. J. et al. Outcomes following transcatheter aortic valve replacement in the united states. JAMA 310, 2069–2077 (2013).
Schymik, G. et al. Long-term results of transapical versus transfemoral tavi in a real world population of 1000 patients with severe symptomatic aortic stenosis. Circ. Cardiovasc. Interv. 8, e000761 (2015).
Blackstone, E. H. et al. Propensity-matched comparisons of clinical outcomes after transapical or transfemoral transcatheter aortic valve replacement: a placement of aortic transcatheter valves (PARTNER)-I trial substudy. Circulation 131, 1989–2000 (2015).
Zhao, A., Minhui, H., Li, X. & Zhiyun, X. A meta-analysis of transfemoral versus transapical transcatheter aortic valve implantation on 30-day and 1-year outcomes. Heart Surg. Forum 18, E161–E166 (2015).
Patel, J. S. et al. Access options for transcatheter aortic valve replacement in patients with unfavorable aortoiliofemoral anatomy. Curr. Cardiol. Rep. 18, 110 (2016).
Cohen, M. G. et al. Transseptal antegrade transcatheter aortic valve replacement for patients with no other access approach — a contemporary experience. Catheter Cardiovasc. Interv. 82, 987–993 (2013).
Greenbaum, A. B. et al. Caval-aortic access to allow transcatheter aortic valve replacement in otherwise ineligible patients: initial human experience. J. Am. Coll. Cardiol. 63, 2795–2804 (2014).
Mylotte, D. et al. Transcarotid transcatheter aortic valve replacement: feasibility and safety. JACC Cardiovasc. Interv. 9, 472–480 (2016).
Gaia, D. F. et al. New braile inovare transcatheter aortic prosthesis: clinical results and follow-up. EuroIntervention 11, 682–689 (2015).
Ribeiro, H. B. et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J. Am. Coll. Cardiol. 62, 1552–1562 (2013).
Blumenstein, J. et al. Challenges of coronary angiography and intervention in patients previously treated by tavi. Clin. Res. Cardiol. 104, 632–639 (2015).
Garcia, E. et al. Transcatheter aortic valve implantation in patients with a mechanical mitral valve [Spanish]. Rev. Esp. Cardiol. 64, 1052–1055 (2011).
Mahadevia, R. et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation 129, 673–682 (2014).
Mylotte, D. et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J. Am. Coll. Cardiol. 64, 2330–2339 (2014).
Yoon, S. H. et al. Transcatheter aortic valve replacement with early- and new-generation devices in bicuspid aortic valve stenosis. J. Am. Coll. Cardiol. 68, 1195–1205 (2016).
Barbanti, M. et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 128, 244–253 (2013).
Roy, D. A. et al. Transcatheter aortic valve implantation for pure severe native aortic valve regurgitation. J. Am. Coll. Cardiol. 61, 1577–1584 (2013).
Testa, L. et al. CoreValve implantation for severe aortic regurgitation: a multicentre registry. EuroIntervention 10, 739–745 (2014).
Seiffert, M. et al. Initial german experience with transapical implantation of a second-generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc. Interv. 7, 1168–1174 (2014).
Wendt, D. et al. Transapical transcatheter aortic valve for severe aortic regurgitation: expanding the limits. JACC Cardiovasc. Interv. 7, 1159–1167 (2014).
Urena, M. et al. Transcatheter aortic valve replacement to treat pure aortic regurgitation on noncalcified native valves. J. Am. Coll. Cardiol. 68, 1705–1706 (2016).
Dvir, D. et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 312, 162–170 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02207569 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02202434 (2017).
Author information
Authors and Affiliations
Contributions
B.M.J. and S.R.K. researched data for the article. B.M.J., A.K., S.M., W.A.J., L.G.S., and S.R.K. contributed substantially to the discussion of content. B.M.J., A.K., and S.R.K. wrote the manuscript. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
E.M.T. has received travel fees from Edwards Lifesciences, and is an unpaid member of the executive committee for the PARTNER trial. W.A.J. has received fees from Edwards Lifesciences for CoreLab work in the PARTNER trial. L.G.S. is an unpaid member of the executive committee for the PARTNER trial. S.R.K. is an unpaid member of the executive committee for the Lotus, Portico, and PARTNER trials. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Jones, B., Krishnaswamy, A., Tuzcu, E. et al. Matching patients with the ever-expanding range of TAVI devices. Nat Rev Cardiol 14, 615–626 (2017). https://doi.org/10.1038/nrcardio.2017.82
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2017.82
This article is cited by
-
In silico biomechanical design of the metal frame of transcatheter aortic valves: multi-objective shape and cross-sectional size optimization
Structural and Multidisciplinary Optimization (2021)
-
Platelet biology and functions: new concepts and clinical perspectives
Nature Reviews Cardiology (2019)
-
Transcatheter aortic valve implantation and surgical aortic valve replacement among hospitalized patients with and without type 2 diabetes mellitus in Spain (2014–2015)
Cardiovascular Diabetology (2017)