Key Points
-
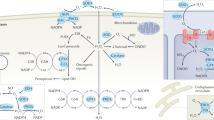
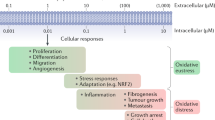
Reactive oxygen species (ROS) produced at the mitochondrial respiratory chain and to a lesser extent by peroxisomes and plasma membrane-bound NADPH oxidases have a crucial role in signalling pathways in the cell, including a requirement for ROS to promote S phase entry.
-
Excess ROS can lead to cell cycle arrest, senescence or cell death and thus proper management of ROS is required to prevent premature ageing and disease.
-
Increased ROS levels in the haematopoietic system lead to stem cell depletion and bone marrow failure and are associated with activation of the retinoblastoma (RB) pathway through induction of the cyclin-dependent kinase inhibitor INK4A.
-
RB is required to maintain the haematopoietic stem cell (HSC) pool and prevent aberrant S phase entry of HSCs in response to proliferative stress.
-
Defects in Rb1-deficient haematopoiesis are due in part to non-cell-autonomous events, including the influence of the bone marrow niche on the development of myeloproliferative disease and the sensitivity of stem cells and erythroid cells to proliferative stress. These observations are indicative of a role for RB in regulating the stromal microenvironment.
-
Disruption of ataxia telangiectasia mutated (ATM) or the Forkhead box O (Foxo) transcription factors in mice leads to increased ROS levels in HSCs that induce p38 mitogen-activated protein kinase activity, INK4A expression, downregulation of N-cadherin and increased stem cell recruitment into the cell cycle, probably due to reduced expression of the cyclin-dependent kinase inhibitors p21 and p27. Thus, ATM and Foxo act upstream of RB in the response of the cell to ROS.
-
Proliferative stress also induces erythroid maturation defects in the Rb1-null mouse that include failure to exit the cell cycle during terminal differentiation, increased ROS levels, increased DNA damage and altered management of mitochondrial mass.
-
Acetylation of RB modulates its rate of turnover in response to oxidative stress and protein phosphatase 2A-dependent dephosphorylation modulates its activity.
-
The role of the RB pathway in responding to oxidative stress in the haematopoietic system has implications for how other cell types respond to ROS, including human tumours that lack a functional RB pathway. Such cancers exhibit defective stress responses and increased levels of ROS, making them more susceptible to chemotherapy-induced death.
Abstract
Exposure to pro-oxidants and defects in the repair of oxidative base damage are associated with disease and ageing and also contribute to the development of anaemia, bone marrow failure and haematopoietic malignancies. This Review assesses emerging data indicative of a specific role for the RB tumour suppressor pathway in the response of the haematopoietic system to oxidative stress. This is mediated through signalling pathways that involve DNA damage sensors, forkhead box O (Foxo) transcription factors and p38 mitogen-activated protein kinases and has downstream consequences for cell cycle progression, antioxidant capacity, mitochondrial mass and cellular metabolism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Almasan, A. et al. Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes and apoptosis. Proc. Natl Acad. Sci. USA 92, 5436–5440 (1995).
Harrington, E. A., Bruce, J. L., Harlow, E. & Dyson, N. pRB plays an essential role in cell cycle arrest induced by DNA damage. Proc. Natl Acad. Sci. USA 95, 11945–11950 (1998).
Knudsen, K. E. et al. RB-dependent S-phase response to DNA damage. Mol. Cell. Biol. 20, 7751–7763 (2000).
Sherr, C. J. & McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2, 103–112 (2002).
Kim, W. Y. & Sharpless, N. E. The regulation of INK4/ARF in Cancer and Aging. Cell 127, 265–275 (2006).
Knudsen, E. S. & Knudsen, K. E. Tailoring to RB: tumour suppressor status and therapeutic response. Nature Rev. Cancer 8, 714–724 (2008).
Cam, H. & Dynlacht, B. D. Emerging roles for E2F: beyond the G1/S transition and DNA replication. Cancer Cell 3, 311–316 (2003).
Giacinti, C. & Giordano, A. RB and cell cycle progression. Oncogene 25, 5220–5227 (2006).
Yu, Q., Ciermeych, M. A. & Sicinski, P. Ras and Myc can drive oncogenic cell proliferation through individual D-cyclins. Oncogene 24, 7114–7119 (2005).
Trimarchi, J. & Lees, J. A. Sibling rivalry in the E2F family. Nature Rev. Mol. Cell Biol. 3, 11–20 (2002).
El-Deiry, W. S. et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 54, 1169–1174 (1994).
Cicchillitti, L., Fasanaro, P., Biglioli, P., Capogrossi, M. C. & Martelli, F. Oxidative stress induces protein phosphatase 2A-dependent dephosphorylation of the pocket proteins pRb, p107 and p130. J. Biol. Chem. 278, 19509–19517 (2003).
Avni, D. et al. Active localization of the retinoblastoma protein in chromatin and its response to S phase DNA damage. Mol. Cell 12, 735–746 (2003).
Havens, C. G., Ho, H., Yoshioka, N. & Dowdy, S. F. Regulation of late G1/S phase transition and APCCdh1 by reactive oxygen. Mol. Cell. Biol. 26, 4701–4711 (2006). Quenching ROS induced a G1 arrest suggesting that endogenous ROS were required for S phase entry. Cell cycle arrest was associated with failure to stabilize cyclin A1, SKP2 or EMI1 and persistent activity of the APC–CDH1 ubiquitin ligase complex.
Beckman, K. B. & Ames, B. N. The free radical theory of aging matures. Physiol. Rev. 78, 547–581 (1998).
Finkel, T. & Holbrook, N. J. Oxidants, oxidative stress and the biology of aging. Nature 408, 239–247 (2000).
Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15, 247–254 (2003).
Balaban, R. S., Nemoto, S. & Finkel, T. Mitochondria, oxidants and aging. Cell 120, 483–495 (2005).
Lambeth, J. D. NOX enzymes and the biology of reactive oxygen. Nature Rev. Cancer 4, 181–189 (2004).
Conour, J. E., Graham, W. V. & Gaskins, H. R. A combined in vitro/bioinformatic investigation of redox regulatory mechanisms governing cell cycle progression. Physiol. Genomics 18, 196–205 (2004).
Menon, S. G. et al. Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res. 63, 2109–2117 (2003).
Menon, S. G. & Goswami, P. C. A redox cycle within the cell cycle: ring in the old with the new. Oncogene, 24 1–9 (2006).
Stubbe, J. & Riggs-Gelasco, P. Harnessing free radicals: formation and function of the tyrosyl radical in ribonucleotide reductase. Trends Biochem. Sci. 23, 438–443 (1998).
Miller, J. J. et al. Emi1 stably binds and inhibits the anaphase-promoting complex/cyclosome as a pseudosubstrate inhibitor. Genes Dev. 20, 2410–2420 (2006).
Janzen, V. et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4A. Nature 443, 421–426 (2006). Showed that INK4A levels increased with age in HSCs and such cells also showed reduced self-renewal and were less effective in homing to and repopulating host bone marrow. Targeted deletion of INK4A in mice improved the transplant potential of ageing stem cells and restored tolerance of cytotoxic agents.
Cheng, T. et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287, 1804–1808 (2000).
Kozar, K. et al. Mouse development and cell proliferation in the absence of D-type cyclins. Cell 118, 477–491 (2003).
Spike, B. T. et al. The Rb tumor suppressor is required for stress erythropoiesis. EMBO J. 23, 4319–4329 (2004). Shows that red cell maturation defects can be induced by acute deletion of Rb1 and haemolytic anaemia in vivo. Rb1 -null haematopoietic tissue is also defective for long-term repopulation resulting in myeloproliferation and bone marrow failure.
Daria, D. et al. The retinoblastoma tumor suppressor is a critical intrinsic regulator for hematopoietic stem and progenitor cells under stress. Blood 111, 1894–1902 (2008). This paper shows that Rb1 -deleted adult HSCs have reduced repopulating potential and increased activity in peripheral tissues. Following challenge with 5-fluorouracil, HSCs from these animals are more often in S phase of cell cycle and there is increased extramedullary haematopoiesis in the spleen.
Walkley, C. R., Shea, J. M., Sims, N. A., Purton, L. E. & Orkin, S. H. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 129, 1081–1095 (2007). The authors showed that myeloproliferative disease in Rb1flox/flox; Mx1 – Cre mice resulted from a failure in synergistic interactions between myeloid cells and the BM niche and was independent of the HSC defect.
Spike, B. T. & Macleod, K. F. The Rb tumor suppressor in stress responses and hematopoietic homeostasis. Cell Cycle 4, e181–e184 (2005).
Orford, K. W. & Scadden, D. T. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nature Rev. Genet. 9, 115–128 (2008).
Fleming, W. H. et al. Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells. J. Cell Biol. 122, 897–902 (1993).
Krishnamurthy, J. et al. p16INK4A induces an age-dependent decline in islet regenerative potential. Nature 443, 453–457 (2006).
Molofsky, A. V. et al. Increasing p16INK4A expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443, 448–452 (2006).
Park, I. et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423, 302–305 (2003).
Lessard, J. & Sauvageau, G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 423, 255–260 (2003).
Williams, B. O. et al. Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J. 13, 4251–4259 (1994).
Robanus-Maandag, E. C. et al. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 13, 4260–4268 (1994).
Whyatt, D. & Grosveld, F. Cell-autonomous function of the retinoblastoma tumor suppressor protein: new interpretations of old phenotypes. EMBO Rep. 3, 130–135 (2002).
Walkley, C. R. Rb is dispensable for self-renewal and multilineage differentiation of adult hematopoietic stem cells. Proc. Natl Acad. Sci. USA 103, 9057–9062 (2006).
Longley, D. B., Harkin, D. P. & Johnston, P. G. 5-fluorouracil: mechanisms of action and clinical strategies. Nature Rev. Cancer 3, 330–338 (2003).
Hosokawa, K. et al. Function of oxidative stress in the regulation of hematopoietic stem cell-niche interaction. Biochem. Biophys. Res. Comm. 363, 578–583 (2007).
Viatour, P. et al. Hematopoietic stem cell quiescence is maintained by compound contributions of the retinoblastoma gene family. Cell Stem Cell (in the press).
Lecouter, J. E. et al. Strain-dependent myeloid hyperplasia, growth deficiency, and accelerated cell cycle in mice lacking the Rb-related p107 gene. Mol. Cell. Biol. 18, 7455–7465 (1998).
Adams, G. B. & Scadden, D. T. The hematopoietic stem cell in its place. Nature Immunol. 7, 333–337 (2006).
Scadden, D. T. The stem-cell niche as an entity of action. Nature 441, 1075–1079 (2006).
Thomas, D. M. et al. Retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol. Cell 8, 303–316 (2001).
Huang, H. & Tindall, D. J. Dynamic FoxO transcription factors. J. Cell Sci. 120, 2479–2487 (2007).
Miyamoto, K. et al. FoxO3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 1, 101–112 (2007). Knockout of Foxo3a in mice revealed a requirement for FOXO3A in HSCs, but not in myeloid progenitors. Foxo3a−/− HSCs were defective for colony formation in vitro and for repopulating capacity in vivo , had increased ROS levels and had reduced expression of SOD2 and catalase.
Tothova, Z. et al. FoxOs are critical mediators of hematological resistance to physiologic oxidative stress. Cell 128, 325–339 (2007). Increased ROS in Foxo-deleted HSCs, but not in myeloid progenitors, led the authors to show that INK4A induction, defective HSC repopulation and cell cycle control could be rescued by treatment of mice with NAC.
Marinkovic, D. et al. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J. Clin. Invest. 117, 2133–2144 (2007). This work shows that loss of FOXO3A sensitizes mice to haemolytic anaemia induced by phenylhydrazine treatment. Loss of FOXO3A causes red blood cells to accumulate increased levels of ROS.
Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of hematopoietic stem cells. Nature 431, 997–1002 (2004). Loss of ATM in the haematopoietic system results in reduced bone marrow cellularity, loss of stem cell self-renewal, increased INK4A expression and increased ROS.
Ito, K. et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic cells. Nature Med. 12, 446–451 (2006). Atm−/− HSCs showed increased p38 activity and inhibition of p38 with NAC, or with chemical inhibitors of p38 prevented induction of INK4A and restored HSC self-renewal.
Wada, T. & Penninger, J. M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23, 2838–2849 (2004).
Matsuzawa, A. & Ichijo, H. Redox control of cell fate by MAP kinase: physiological roles of ASK1–MAP kinase pathway in stress signaling. Biochim. Biophys. Acta 1780, 1325–1336 (2008).
Ohtani, N. et al. Opposing effects of Ets and Id proteins on p16INK4A expression during cellular senescence. Nature 409, 1067–1070 (2001).
Tsai, W. B., Chung, Y. L., Takahashi, Y., Xu, Z. & Hu, M. C. T. Functional interaction between FOXO3a and ATM regulates DNA damage response. Nature Cell Biol. 10, 460–467 (2008).
Bakkenist, C. J. & Kastan, M. B. Initiating cellular stress responses. Cell 118, 9–17 (2004).
Kastan, M. B. & Bartek, J. Cell-cycle checkpoints and cancer. Nature 432, 316–323 (2004).
Polager, S., Kalma, Y., Berkovich, E. & Ginsberg, D. E2Fs up-regulate expression of genes involved in DNA replication, DNA repair and mitosis. Oncogene 21, 437–446 (2002).
Ren, B. et al. E2F integrates cell cycle progression with DNA repair, replication and G2/M checkpoints. Genes Dev. 16, 245–256 (2002).
Hoskins, E. E. et al. Coordinate regulation of Fanconi anemia gene expression occurs through the Rb/E2F pathway. Oncogene 28 Apr 2008 (doi: 10.1038/onc.2008.121).
D'Andrea, A. D. & Grompe, M. The Fanconi Anemia/BRCA pathway. Nature Rev. Cancer 3, 23–34 (2003).
O'Driscoll, M., Ruiz-Perez, V. L., Woods, C. G., Jeggo, P. A. & Goodship, J. A. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nature Genet. 33, 497–501 (2003).
Venkitaraman, A. R. Tracing the network connecting BRCA and Fanconi anaemia proteins. Nature Rev. Cancer 4, 266–276 (2004).
Resnick, I. B. et al. Nijmegen breakage syndrome: clinical characteristics and mutation analysis in eight unrelated Russian families. J. Pediatr. 140, 355–361 (2002).
Takeuchi, T. & Morimoto, K. Increased formation of 8-hydroxydeoxyguanosine, an oxidative DNA damage in lymphoblasts from Fanconi's anemia patients due to possible catalase deficiency. Carcinogenesis 14, 1115–1120 (1993).
Clarke, A. A., Philpott, N. J., Gordon-Smith, E. C. & Rutherford, T. R. The sensitivity of Fanconi anaemia group C cells to apoptosis induced by mitomycin C is due to oxygen radical generation, not DNA cross-linking. Br. J. Haematol. 96, 240–247 (1997).
Park, S. J. et al. Oxidative stress/damage induces multimerization and interaction of Fanconi Anemia proteins. J. Biol. Chem. 279, 30053–30059 (2004).
Schindler, D. & Hoehn, H. Fanconi anemia mutation causes cellular susceptibility to ambient oxygen. Am. J. Hum. Genet. 43, 429–435 (1988).
Bouchard, V. J., Rouleau, M. & Poirier, G. G. PARP-1, a determinant of cell survival in response to DNA damage. Exp. Hematol. 31, 446–454 (2003).
Barzilai, A., Rotman, G. & Shiloh, Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair 1, 3–25 (2002).
Tsantes, A. E., Bonovas, S., Travlou, A. & Sitaras, N. Redox imbalance, macrocytosis, and RBC homeostasis. Antioxid. Redox Signal. 8, 1205–1216 (2006).
Neumann, C. A. et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour supression. Nature 424, 561–565 (2003).
Lee, T. H. et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 101, 5033–5038 (2003).
Chan, J. Y., Kwong, M., Lo, M., Emerson, R. & Kuypers, F. A. Reduced oxidative stress response in red blood cells from p45NFE2-deficient mice. Blood 97, 2151–2158 (2001).
Lan, Q. et al. Hematotoxicity in workers exposed to low levels of benzene. Science 306, 1774–1776 (2004).
Rothman, N. et al. Benzene poisoning, a risk factor for hematological malignancy, is associated with the NQO1 609C→T mutation and rapid fractional excretion of chlorzoxazone. Cancer Res. 57, 2839–2842 (1997).
Hattangadi, S. M. & Lodish, H. F. Regulation of erythrocyte lifespan: do reactive oxygen species set the clock? J. Clin. Invest. 117, 2075–2077 (2007).
Clark, A. J., Doyle, K. M. & Humbert, P. O. Cell intrinsic functions of pRb in erythropoiesis. Blood 104, 1324–1326 (2004).
Kinross, K. M., Clark, A. J., Iazzolino, R. M. & Humbert, P. O. E2f4 regulates fetal erythropoiesis through the promotion of cellular proliferation. Blood 108, 886–895 (2006).
Dirlam, A., Spike, B. T. & Macleod, K. F. De-regulated E2f-2 activity underlies cell cycle and maturation defects in Rb null erythroblasts. Mol. Cell. Biol. 27, 8713–8728 (2007).
Cam, H. et al. A common set of gene regulatory networks links metabolism and growth inhibition. Mol. Cell 16, 399–411 (2004). This work identifies overlapping sets of target genes regulated by E2F4 and NRF1.
Kelly, D. P. & Scarpulla, R. C. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 18, 357–368 (2004).
Tracy, K. et al. BNIP3 is a RB/E2F target gene required for hypoxia-induced autophagy. Mol. Cell. Biol. 27, 6229–6242 (2007). The authors identify BNIP3 as a direct transcriptional target of RB–E2f and show a functional interaction with HIF at the BNIP3 promoter. Loss of RB increases BNIP3 expression and induces cell death associated with defective autophagy.
Bindra, R. S. et al. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 65, 11597–11604 (2006).
Schlisio, S., Halperin, T., Vidal, M. & Nevins, J. R. Interaction of YY1 with E2Fs, mediated by RYBP, provides a mechanism for specificity of E2F function. EMBO J. 21, 5775–5786 (2002).
Giangrande, P. H., Hallstrom, T. C., Tunyaplin, C., Calame, K. & Nevins, J. R. Identification of E-box factor TFE3 as a functional partner for the E2F3 transcription factor. Mol. Cell Biol. 23, 3707–3720 (2003).
Giangrande, P. H., Zhu, W., Rempel, R. E., Laasko, N. & Nevins, J. R. Combinatorial gene control involving E2F and E box family members. EMBO J. 23, 1336–1347 (2004).
Sankaran, V. G., Orkin, S. H. & Walkley, C. R. Rb instrinsically promotes erythropoiesis by coupling cell cycle exit with mitochondrial biogenesis. Genes Dev. 22, 463–475 (2008). The authors show that Rb1 -null erythroblasts have uncoupled mitochondrial mass regulation from other aspects of terminal maturation, such as cell cycle exit and expression of key maturation markers.
Schweers, R. L. et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc. Natl Acad. Sci. USA 104, 19500–19505 (2007).
Sandoval, H. et al. Essential role for Nix in autophagic maturation of red cells. Nature 454, 232–235 (2008).
Wang, C. et al. Cyclin D1 repression of nuclear respiratory factor 1 integrates nuclear DNA synthesis and mitochondrial function. Proc. Natl Acad. Sci. USA 103, 11567–11572 (2006).
Zhang, H. et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of c-Myc activity. Cancer Cell 11, 407–420 (2007). The authors present data proposing that one method of coping with hypoxia is to reduce mitochondrial mass by inhibiting mitochondrial biogenesis.
Zhang, H. et al. Mitochondrial autophagy is a HIF-1 dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 283, 10892–10903 (2008). The authors present data suggesting that BNIP3 promotes mitophagy by titrating the inhibitory activity of BCL2 away from the crucial autophagy regulator beclin 1.
Tracy, K. & Macleod, K. F. Regulation of mitochondrial integrity, autophagy and cell survival by BNIP3. Autophagy 3, 616–619 (2007).
Hamacher-Brady, A. et al. Response to myocardial ischemia/reperfusion injury involves BNip3 and autophagy. Cell Death Diff. 13, 1–12 (2006).
Mammucari, C. et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metabolism 6, 458–471 (2007).
Polager, S., Ofir, M. & Ginsberg, D. E2F1 regulates autophagy and the transcription of autophagy genes. Oncogene 27, 4860–4864 (2008).
Altiok, S., Xu, M. & Spiegelman, B. M. PPARγ induces cell cycle withdrawal: inhibition of E2F/DP DNA-binding activity via down-regulation of PP2A. Genes Dev. 11, 1987–1998 (1997).
Fajas, L. et al. E2Fs regulate adipocyte differentiation. Dev. Cell 3, 39–49 (2002).
Scime, A. et al. Rb and p107 regulate pre-adipocyte differentiation into white versus brown fat through repression of PGC-1a. Cell Metab. 2, 283–295 (2005).
Esposito, F., Russo, L., Russo, T. & Cimino, F. Retinoblastoma protein dephosphorylation is an early event of cellular response to pro-oxidant conditions. FEBS Lett. 470, 211–215 (2000).
Magenta, A. F., P., Romani, S., Di Stefano, V., Capogrossi, M. C. & Martelli, F. PP2A–PR70 interacts with pRb and mediates its de-phosphorylation. Mol. Cell. Biol. 28, 873–882 (2008).
Yamauchi, A. & Bloom, E. T. Control of cell cycle progression in human natural killer cells through redox regulation of expression and phosphorylation of retinoblastoma gene product protein. Blood 89, 4092–4099 (1997).
Guardavaccaro, D. & Pagano, M. Stabilizers and destabilizers controlling cell cycle oscillators. Mol. Cell 22, 1–4 (2006).
Tedesco, D., Lukas, J. & Reed, S. I. The pRb-related protein p130 is regulated by phosphorylatiton-dependent proteolysis via the protein-ubiquitin ligase SCFSkp2. Genes Dev. 16, 2946–2957 (2002).
Ji, P. et al. An Rb–Skp2–p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant. Mol. Cell 16, 47–58 (2004).
Binne, U. K. et al. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nature Cell Biol. 9, 225–232 (2006).
Wu, C., Miloslavskaya, I., Demontis, S., Maestro, R. & Galaktionov, K. Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature 432, 640–645 (2005).
Chan, H. M., Krstic-Demonacos, M., Smith, L., Demonacos, C. & La Thangue, N. Acetylation control of the retinoblastoma tumour suppressor protein. Nature Cell Biol. 3, 667–674 (2001).
Nguyen, D. X., Baglia, L. A., Huang, S. M., Baker, C. M. & McCance, D. J. Acetylation regulates the differentiation-specific functions of the retinoblastoma protein. EMBO J. 23, 1609–1618 (2004).
Wong, S. & Weber, S. Deacteylation of the retinoblastoma tumour suppressor protein by SIRT1. Biochem. J. 407, 451–460 (2007).
Luo, J. et al. Negative control of p53 by Sir2a promotes cell survival under stress. Cell 107, 137–148 (2001).
Vaziri, H. et al. hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 (2001).
Rodgers, J. T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1a and SIRT1. Nature 434, 113–118 (2005).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1a. Cell 127, 1109–1122 (2006).
Fulco, M. et al. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol. Cell 12, 51–62 (2003).
Puri, P. L. et al. Class 1 histone deacetylases sequentially interact with MyoD and pRb during skeletal myogenesis. Mol. Cell 8, 885–897 (2001).
Di Carlo, A. et al. Hypoxia inhibits myogenic differentiation through accelerated MyoD degradation. J. Biol. Chem. 279, 16332–16338 (2004).
Bakker, W. J., Harris, I. S. & Mak, T. W. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol. Cell 28, 941–953 (2007). CITED2 is an inhibitor of p300–CBP and feeds back to inhibit p300-dependent transactivation by HIF. Induction of CITED2 by hypoxia and by FOXO3A is HIF-dependent. Knockdown of FOXO3 sensitized tumour cells to hypoxia-induced cell death.
Parmar, K., Mauch, P., Vergillo, J. A., Sackstein, R. & Down, J. D. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl Acad. Sci. USA 104, 5431–5436 (2007).
Danet, G. H., Pan, Y., Luongo, J. L., Bonnet, D. A. & Simon, M. C. Expansion of human SCID-repopulating cells under hypoxic conditions. J. Clin. Invest. 112, 126–135 (2003).
Gustaffson, M. V. et al. Hypoxia requires Notch signaling to maintain the undifferentiated cell state. Dev. Cell 9, 617–628 (2005).
Lee, A. C. et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 274, 7936–7940 (1999).
Di Mocco, R. et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444, 638–642 (2006).
Bartkova, J. et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633–637 (2006).
Narita, M. et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003).
Dolado, I. et al. p38α MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell 11, 191–205 (2007). Upregulation of glutathione S -transferase m2 ( Gstm2 ) in HRAS-transformed cells is p38α-dependent. Overexpression of Gstm2 limited p38α activation and desensitized cells to ROS-induced apoptosis.
Gauthier, M. L. et al. Abrogated response to cellular stress identifies DCIS associated with subsequent tumor events and defines basal-like breast tumors. Cancer Cell 12, 479–491 (2007). Shows that overexpression of COX2 induced INK4A expression and growth arrest. This arrest is RB-dependent and, conversely, COX2 expression is induced by inactivation of RB.
Burkhart, D. L. & Sage, J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nature Rev. Cancer 8, 671–682 (2008).
Kim, H. et al. Clostridium difficile toxin A regulates inducible cyclooxygenase-2 and prostaglandin E2 synthesis in colonocytes via reactive oxygen species and activation of p38 MAPK. J. Biol. Chem. 280, 21237–21245 (2005).
Gauthier, M. L. et al. p38 regulates cyclooxygenase-2 in human mammary epithelial cells and is activated in pre-malignant tissue. Cancer Res. 65, 1792–1799 (2005).
Zha, S., Yegnasubramanian, V., Nelson, W. G., Isaacs, W. B. & De Marzo, A. Cyclo-oxygenases in cancer: progress and perspective. Cancer Lett. 215, 1–20 (2004).
Schumacker, P. T. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10, 175–176 (2006).
Trachootham, D. et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocynate. Cancer Cell 10, 241–252 (2006).
Weinberg, R. A. The retinoblastoma protein and cell cycle control. Cell 81, 323–330 (1995).
Wikemheiser-Brokamp, K. A. Retinoblastoma family proteins: insights gained through genetic manipulation of mice. Cell. Mol. Life Sci. 63, 767–780 (2006).
Bremner, R. et al. Direct transcriptional repression by Rb and its reversal by specific cyclins. Mol. Cell. Biol. 15, 3256–3265 (1995).
Weintraub, S. J. et al. Mechanisms of active transcriptional repression by Rb. Nature 375, 812–815 (1995).
Brehm, A. & Kouzarides, T. Retinoblastoma protein meets chromatin. Trends Biochem. Sci. 24, 142–145 (1999).
Hernando, E. et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature 430, 797–802 (2004).
Mittnacht, S. Control of pRB phosphorylation. Curr. Opin. Genet. Dev. 8, 21–27 (1998).
Sage, J., Miller, A. L., Perez-Mancera, P. A., Wysocki, J. M. & Jacks, T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 424, 223–228 (2003).
Classon, M. & Harlow, E. The retinoblastoma tumour suppressor in development and cancer. Nature Rev. Cancer 2, 910–917 (2002).
Chau, B. N. & Wang, J. Y. J. Coordinated regulation of life and death by RB. Nature Rev. Cancer 3, 130–138 (2003).
Guzy, R. D. & Schumacker, P. T. Oxygen sensing by mitochondria at Complex III: the paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 91, 807–819 (2006).
Deng, Y., Chan, S. S. & Chang, S. Telomere dysfunction and tumor suppression: the senesence connection. Nature Rev. Cancer 8, 450–458 (2008).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Wu, L. et al. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature 421, 942–947 (2003).
Wenzel, P. L. et al. Rb is critical in a mammalian tissue stem cell population. Genes Dev. 21, 85–97 (2007).
Lipinski, M., Macleod, K. F., Crowley, D., Mullaney, T. & Jacks, T. Cell-autonomous and non-cell autonomous developmental functions of the retinoblastoma tumor suppressor gene in vivo. EMBO J. 20, 3402–3413 (2001).
Spike, B. T. & Macleod, K. F. Effects of hypoxia on heterotypic macrophage interactions. Cell Cycle 6, 2620–2624 (2007).
Spike, B. T., Dibling, B. C. & Macleod, K. F. Hypoxic stress underlies defects in erythroblast island formation in the Rb null mouse. Blood 110, 2173–2181 (2007).
Iavarone, A. et al. Retinoblastoma promotes definitive erythropoiesis by repressing Id2 in fetal liver macrophages. Nature 432, 1040–1045 (2004).
Adams, G. B. et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603 (2006).
Krug, U., Ganser, A. & Koeffler, H. P. Tumor suppressor genes in normal and malignant hematopoiesis. Oncogene 21, 3475–3495 (2002).
Dickson, C. et al. Amplification of chromosome band 11q13 and a role for cyclin D1 in human breast cancer. Cancer Lett. 90, 43–50 (1995).
Foster, C. S. et al. Transcription factor E2F3 overexpressed in prostate cancer independently predicts clinical outcome. Oncogene 23, 5871–5879 (2004).
Oeggerli, M. et al. E2F3 is the main target gene of the 6p22 amplicon with high specificity for human bladder cancer. Oncogene 25, 6538–6543 (2006).
Moroni, M. C. et al. Apaf-1 is a transcriptional target for E2F and p53. Nature Cell Biol. 3, 552–558 (2001).
Nahle, Z. et al. Direct coupling of the cell cycle and cell death machinery by E2F. Nature Cell Biol. 4, 859–864 (2002).
Hallstrom, T., Mori, S. & Nevins, J. R. An E2F-1 dependent gene expression program that determines the balance betweeen proliferation and cell death. Cancer Cell 13, 11–22 (2008). Expression profiling was used to identify pro-apoptotic E2F1 target genes that are repressed by phosphoinositide 3-kinase and identified AMPKα2, CYO26B1, SOS2 and GRB7, amongst others.
Hershko, T. & Ginsberg, D. Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F-1 mediates apoptosis. J. Biol. Chem. 279, 8627–8634 (2004).
Brugarolas, J., Bronson, R. T. & Jacks, T. p21 is a critical Cdk2 regulator essential for proliferation control in Rb-deficient cells. J. Cell. Biol. 141, 503–514 (1998).
Smith, K. S. et al. Bmi-1 regulation of INK4A–ARF is a downstream requirement for transformation of hematopoietic progenitors by E2a–Pbx1. Mol. Cell 12, 393–400 (2003).
Gilley, J., Coffer, P. J. & Ham, J. FoxO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J. Cell. Biol. 162, 613–622 (2003).
Kranc, K. R. et al. Transcriptional coactivator Cited2 induces Bmi1 and Mel18 and controls fibroblast proliferation via INk4a/ARF. Mol. Cell. Biol. 23, 7658–7666 (2003).
Hershko, T., Korotayev, K., Polager, S. & Ginsberg, D. E2F-1 modulates p38 MAPK phosphorylation via transcriptional regulation of Ask1 and Wip1. J. Biol. Chem. 281, 31309–31316 (2006).
Nowak, K., Killmer, K., Gessner, C. & Lutz, W. E2F-1 regulates expression of FOXO1 and FOXO3a. Biochim. Biophys. Acta 1769, 244–252 (2007).
Tanaka, H. et al. E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kB activity that facilitates MnSOD-mediated ROS elimination. Mol. Cell 9, 1017–1029 (2002).
Rius, J. et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 453, 807–811 (2008).
Chen, J. et al. Generation of normal lymphocyte populations of Rb-deficient ES cells: analysis of gene function by RAG-2-deficient blastocyst complementation. Curr. Biol. 3, 405–413 (1993).
Lasorella, A., Noseda, M., Beyna, M. & Iavarone, A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature 407, 592–598 (2000).
de Bruin, A. et al. Rb function in extraembryonic tissue lineages suppresses apoptosis in the CNS of Rb-deficient mice. Proc. Natl Acad. Sci. USA 100, 6546–6551 (2003).
Ye, M. et al. Hematopoietic stem cells expressing the myeloid lysozyme gene retain long-term, multi-lineage repopulation potential. Immunity 19, 689–699 (2003).
Acknowledgements
The author is grateful to the J. P. McCarthy Foundation, the Aplastic Anaemia and MDS International Foundation and the National Heart Lung & Blood Institute (RO1 HL080262) for funding of work in her laboratory relating to oxidative stress, erythropoiesis and haematopoietic diseases. The author also gratefully acknowledges the experimental skills and intellectual input to work in the laboratory in the related research area by current laboratory members K. Tracy, J. Knabb and D. Glick and by former graduate students in the laboratory B. T. Spike and A. Dirlam.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
National Cancer Institute
National Cancer Institute Drug Dictionary
OMIM
FURTHER INFORMATION
Glossary
- Haematopoietic stem cell
-
A cell residing at the apex of the cellular hierarchy in the haematopoietic system that gives rise to all other lineages and cell types in the blood system.
- Non-cell-autonomous
-
Effects in tissues that are the consequence of a defect or altered phenotype in a different tissue or cell type.
- Vav–Cre
-
Expression of the Cre recombinase under the control of the Vav promoter. which is restricted in its expression to haemangioblast-derived cell types. Thus Vav–Cre drives deletion of floxed alleles in embryonic and adult haematopoietic stem cells and their progeny, as well as in endothelial cells.
- Poly(I)poly(C)
-
A form of double-stranded RNA that is a potent inducer of interferon and is used to activate the Mx1 promoter driving Cre recombinase expression.
- Mx1–Cre
-
Expression of the Cre recombinase under the control of the silent Mx1 gene promoter: Cre is not expressed unless the transgenic mouse is challenged with agents that induce the interferon response, such as poly(I)poly(C). Expression is high in the haematopoietic system, liver and kidneys and lower in other cell types tested.
- N-cadherin
-
A member of the cadherin family of cell–cell adhesion proteins bearing conserved structural motifs known as 'cadherin repeats'. N-cadherin is highly expressed in mature neurons.
- Repopulating capacity
-
The ability of haematopoietic tissue and cells to regenerate the blood system when transplanted into host animals that have had their bone marrow ablated through exposure to lethal or sublethal doses of ionizing irradiation or to cytotoxic drugs.
- Myeloproliferation
-
Expansion of myeloid elements within the blood system.
- Osteoclasts
-
Myeloid-derived cells within the bone marrow niche that interact with bone matrix and osteoblasts to influence stem cell development.
- Antioxidants
-
Compounds and enzymes that neutralize ROS by accepting electrons from free radicals.
- Fanconi anaemia
-
A cancer susceptibility syndrome in which genetically predisposed individuals are sensitized to DNA crosslinking agents, experience bone marrow failure and anaemia and show varying degrees of developmental abnormalities.
- Poly(ADP-ribose)
-
A polymer generated by and conjugated to target proteins by members of the poly(ADP)-ribose polymerase resulting in increased negative charge and altered activities of modified proteins.
- Pentose phosphate pathway
-
A series of enzymatic reactions in which NADPH is produced in cells by conversion of glucose-6-phsophate to ribulose-5-phosphate.
- Fenton reaction
-
The oxidation of Fe2+ to generate Fe3+ and highly reactive hydroxyl radicals.
- Ischaemia
-
Deprivation or insufficiency of blood supply to tissues associated with hypoxia and nutrient deprivation, frequently with necrotic cell death.
- Endosteal surface
-
The inner surface of bone bordering the bone marrow cavity.
- Ki67
-
Ki67 is a marker of proliferation that may have a role in ribosome biogenesis. Immunohistochemical staining for Ki67 on tumour sections and tissues is commonly used to mark out proliferating cells in situ.
- Cyclooxygenase 2
-
(COX2). An enzyme with both peroxidase and dioxygenase activity involved in synthesis of prostaglandins from arachadonic acid, COX2 is activated by inflammation and upregulated in colorectal, breast and other cancers. COX2 inhibitors are used as anti-inflammatory drugs.
Rights and permissions
About this article
Cite this article
Macleod, K. The role of the RB tumour suppressor pathway in oxidative stress responses in the haematopoietic system. Nat Rev Cancer 8, 769–781 (2008). https://doi.org/10.1038/nrc2504
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2504
This article is cited by
-
Interplay of oxidative stress, cellular communication and signaling pathways in cancer
Cell Communication and Signaling (2024)
-
Motifs enable communication efficiency and fault-tolerance in transcriptional networks
Scientific Reports (2020)
-
A novel bone marrow targeted gadofullerene agent protect against oxidative injury in chemotherapy
Science China Materials (2017)
-
Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer
Nature Immunology (2013)
-
Redox regulation in stem-like cancer cells by CD44 variant isoforms
Oncogene (2013)