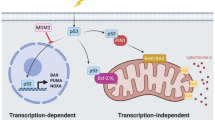
Abstract
Recent findings show that even the brief inactivation of a single oncogene might be sufficient to result in the sustained loss of a neoplastic phenotype. It is therefore possible that the targeted inactivation of oncogenes could be a specific and effective treatment for cancer. So why does oncogene inactivation cause tumour regression and will this be a generally successful approach for the treatment of human neoplasia?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chin, L. et al. Essential role for oncogenic Ras in tumour maintenance. Nature 400, 468–472 (1999).
Felsher, D. W. & Bishop, J. M. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell 4, 199–207 (1999).
Jain, M. et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science 297, 102–104 (2002).
Pelengaris, S., Littlewood, T., Khan, M., Elia, G. & Evan, G. Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol. Cell 3, 565–577 (1999).
Pelengaris, S., Khan, M. & Evan, G. I. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell 109, 321–334 (2002).
Huettner, C. S., Zhang, P., Van Etten, R. A. & Tenen, D. G. Reversibility of acute B-cell leukaemia induced by BCR–ABL1. Nature Genet. 24, 57–60 (2000).
D'Cruz, C. M. et al. c-MYC induces mammary tumorigenesis by means of a preferred pathway involving spontaneous Kras2 mutations. Nature Med. 7, 235–239 (2001).
Fisher, G. H. et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 15, 3249–3262 (2001).
Sawyers, C. L. Disabling Abl-perspectives on Abl kinase regulation and cancer therapeutics. Cancer Cell 1, 13–15 (2002).
Sawyers, C. L. Finding the next Gleevec: FLT3 targeted kinase inhibitor therapy for acute myeloid leukemia. Cancer Cell 1, 413–415 (2002).
Weinstein, I. B. Cancer. Addiction to oncogenes: the Achilles heal of cancer. Science 297, 63–64 (2002).
Gossen, M. et al. Transcriptional activation by tetracyclines in mammalian cells. Science 268, 1766–1769 (1995).
Kistner, A. et al. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc. Natl Acad. Sci. USA 93, 10933–10938 (1996).
Karlsson, A. et al. Genomically complex lymphomas undergo sustained tumor regression upon MYC inactivation unless they acquire novel chromosomal translocations. Blood 101, 2797–2803.
Ewald, D. et al. Time-sensitive reversal of hyperplasia in transgenic mice expressing SV40 T antigen. Science 273, 1384–1386 (1996).
Felsher, D. W. & Bishop, J. M. Transient excess of MYC activity can elicit genomic instability and tumorigenesis. Proc. Natl Acad. Sci. USA 96, 3940–3944 (1999).
Gunther, E. J. et al. Impact of p53 loss on reversal and recurrence of conditional Wnt-induced tumorigenesis. Genes Dev. 17, 488–501 (2003).
Druker, B. J. et al. Efficacy and safety of a specific inhibitor of the BCR–ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344, 1031–1037 (2001).
Kantarjian, H. et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N. Engl. J. Med. 346, 645–652 (2002).
Talpaz, M. et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood 99, 1928–1937 (2002).
Demetri, G. D. et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 347, 472–480 (2002).
Gorre, M. E. & Sawyers, C. L. Molecular mechanisms of resistance to STI571 in chronic myeloid leukemia. Curr. Opin. Hematol. 9, 303–307 (2002).
Shah, N. P. et al. Multiple BCR–ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2, 117–125 (2002).
Greaves, M. F. Differentiation-linked leukemogenesis in lymphocytes. Science 234, 697–704 (1986).
Holtzer, H., Biehl, J., Yeoh, G., Meganathan, R. & Kaji, A. Effect of oncogenic virus on muscle differentiation. Proc. Natl Acad. Sci. USA 72, 4051–4055 (1975).
Boettiger, D., Soltesz, R., Holtzer, H. & Pacifici, M. Infection of chick limb bud presumptive chondroblasts by a temperature-sensitive mutant of Rous sarcoma virus and the reversible inhibition of their terminal differentiation in culture. Mol. Cell. Biol. 3, 1518–1526 (1983).
Esteller, M. et al. Cancer epigenetics and methylation. Science 297, 1807–1808; discussion 1807–1808 (2002).
Rhee, I. et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature 416, 552–556 (2002).
Jang, M. et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275, 218–220 (1997).
Okamoto, A. et al. Mutations and altered expression of p16INK4 in human cancer. Proc. Natl Acad. Sci. USA 91, 11045–11049 (1994).
Berman, D. M. et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 297, 1559–1561 (2002).
Di Croce, L. et al. Methyltransferase recruitment and DNA hypermethylation of target promoters by an oncogenic transcription factor. Science 295, 1079–1082 (2002).
Ogawa, H., Ishiguro, K., Gaubatz, S., Livingston, D. M. & Nakatani, Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 296, 1132–1136 (2002).
Bakin, A. V. & Curran, T. Role of DNA 5-methylcytosine transferase in cell transformation by fos. Science 283, 387–390 (1999).
Baudino, T. A. et al. c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev. 16, 2530–2543 (2002).
Huang, M. E. et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 72, 567–572 (1988).
Sanz, M. A., Martin, G. & Diaz-Mediavilla, J. All-trans-retinoic acid in acute promyelocytic leukemia. N. Engl. J. Med. 338, 393–394 (1998).
Acknowledgements
Thank you to the members of my laboratory for a critical reading of the manuscript, and to H. Varmus and G. Klein for kindly bringing relevant literature to my attention. D.W.F. is supported by grants from the National Institutes of Health, the Lymphoma Research Foundation, the Elsa Pardee Foundation and the Esther Ehrman Lazard Faculty Scholar Award.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Cancer.gov
LocusLink
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Felsher, D. Cancer revoked: oncogenes as therapeutic targets. Nat Rev Cancer 3, 375–379 (2003). https://doi.org/10.1038/nrc1070
Issue Date:
DOI: https://doi.org/10.1038/nrc1070
This article is cited by
-
Near-infrared light triggered in situ release of CO for enhanced therapy of glioblastoma
Journal of Nanobiotechnology (2023)
-
To metabolomics and beyond: a technological portfolio to investigate cancer metabolism
Signal Transduction and Targeted Therapy (2023)
-
Molecular radio afterglow probes for cancer radiodynamic theranostics
Nature Materials (2023)
-
Structure and Photosensitaizer Ability of Polymethine Dyes in Photodynamic Therapy: A Review
Theoretical and Experimental Chemistry (2023)
-
MYC oncogene elicits tumorigenesis associated with embryonic, ribosomal biogenesis, and tissue-lineage dedifferentiation gene expression changes
Oncogene (2022)