Abstract
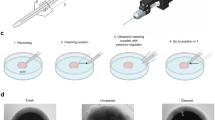
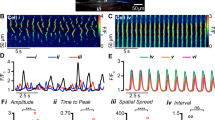
The study of enteric neurons is key to understanding intestinal motility and crucial to the design of therapeutic strategies for dealing with neurogenic disorders. However, enteric neurons have historically been inaccessible to patch-clamp recording. We report here the first technique that allows patch-clamp recording of neurons from the intact myenteric plexus of the mouse duodenum. The mucosa, submucosa and circular muscles are removed, exposing the myenteric plexus on the longitudinal muscle. Proteolytic treatment of exposed ganglia combined with gentle cell-surface cleaning allows gigaseal formation. Compared with previous studies using intracellular microelectrode recordings or cultured myenteric neurons, this technique provides an opportunity to explore properties of single or multiple ion channels in myenteric neurons in their native environment. The protocol—from the tissue preparation to patch-clamp recording—can be completed in ∼4 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
09 June 2011
In the version of this article initially published, two important references were omitted in the discussion of the development of this method. In the fifth paragraph of the Introduction (at the top of page 16), the following sentence has been inserted before "This technique requires...": There were early demonstrations of patch clamping of myenteric neurons in the guinea pig51 and mouse52. The corresponding references are listed below: 51. Kunze, W. et al. The soma and neurites of primary afferent neurons in the guinea-pig intestine respond differently to deformation. J. Physiol. 526, 375–385 (2000). 52. Mao, Y., Wang, B. & Kunze, W. Characterization of myenteric sensory neurons in the mouse small intestine. J. Neurophysiol. 96, 998–1010 (2006). This information has been added to the HTML and PDF versions of the article.
22 October 2018
In the HTML version of this article published online, the abstract contains a typo in the first sentence: "key to understanding intestinal motility anGutn of therapeutic strategies" should read "key to understanding intestinal motility and crucial to the design of therapeutic strategies." The PDF version of the article is correct.
References
Furness, J.B., Kunze, W.A. & Clerc, N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am. J. Physiol. 277, G922–928 (1999).
Furness, J.B. The Enteric Nervous System (Blackwell, Oxford, 2006).
Wood, J.D. Enteric nervous system: sensory physiology, diarrhea and constipation. Curr. Opin. Gastroenterol. 26, 102–108 (2010).
Brookes, S. Retrograde tracing of enteric neuronal pathways. Neurogastroenterol. Motil. 13, 1–18 (2001).
Furness, J.B. The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterol. Motil. 20 (Suppl 1): 32–38 (2008).
Wood, J.D. Enteric nervous system: reflexes, pattern generators and motility. Curr. Opin. Gastroenterol. 24, 149–158 (2008).
Nurgali, K. Plasticity and ambiguity of the electrophysiological phenotypes of enteric neurons. Neurogastroenterol. Motil. 21, 903–913 (2009).
Rugiero, F. et al. Analysis of whole-cell currents by patch clamp of guinea-pig myenteric neurones in intact ganglia. J. Physiol. 538, 447–463 (2002).
Rugiero, F. et al. Selective expression of a persistent tetrodotoxin-resistant Na+ current and NaV1.9 subunit in myenteric sensory neurons. J. Neurosci. 23, 2715–2725 (2003).
Hirst, G.D., Holman, M.E. & Spence, I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J. Physiol. 236, 303–326 (1974).
Nishi, S. & North, R.A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J. Physiol. 231, 471–491 (1973).
Furness, J.B., Kunze, W.A., Bertrand, P.P., Clerc, N. & Bornstein, J.C. Intrinsic primary afferent neurons of the intestine. Prog. Neurobiol. 54, 1–18 (1998).
Kunze, W.A. & Furness, J.B. The enteric nervous system and regulation of intestinal motility. Annu. Rev. Physiol. 61, 117–142 (1999).
Galligan, J.J., LePard, K.J., Schneider, D.A. & Zhou, X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J. Auton. Nerv. Syst. 81, 97–103 (2000).
Morita, K., North, R.A. & Tokimasa, T. The calcium-activated potassium conductance in guinea-pig myenteric neurons. J. Physiol. 329, 341–354 (1982).
Hirst, G.D., Johnson, S.M. & van Helden, D.F. The slow calcium-dependent potassium current in a myenteric neuron of the guinea-pig ileum. J. Physiol. 361, 315–337 (1985).
North, R.A. & Tokimasa, T. Persistent calcium-sensitive potassium current and the resting properties of guinea-pig myenteric neurons. J. Physiol. 386, 333–353 (1987).
Vogalis, F., Furness, J.B. & Kunze, W.A. After hyperpolarization current in myenteric neurons of the guinea pig duodenum. J. Neurophysiol. 85, 1941–1951 (2001).
Bornstein, J.C., Costa, M., Furness, J.B. & Lees, G.M. Electrophysiology and enkephalin immunoreactivity of identified myenteric plexus neurones of guinea-pig small intestine. J. Physiol. 351, 313–325 (1984).
Kunze, W.A., Furness, J.B., Bertrand, P.P. & Bornstein, J.C. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. J. Physiol. 506, 827–842 (1998).
Neher, E., Sakmann, B. & Steinbach, J.H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 375, 219–228 (1978).
Hamill, O.P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391, 85–100 (1981).
Franklin, J.L. & Willard, A.L. Voltage-dependent sodium and calcium currents of rat myenteric neurons in cell culture. J. Neurophysiol. 69, 1264–1275 (1993).
Starodub, A.M. & Wood, J.D. Selectivity of omega-CgTx-MVIIC toxin from Conus magus on calcium currents in enteric neurons. Life Sci. 64, PL305–10 (1999).
Zholos, A.V., Baidan, L.V., Starodub, A.M. & Wood, J.D. Potassium channels of myenteric neurons in guinea-pig small intestine. Neuroscience 89, 603–618 (1999).
Zhou, X. & Galligan, J.J. Non-additive interaction between nicotinic cholinergic and P2X purine receptors in guinea-pig enteric neurons in culture. J. Physiol. 513, 685–697 (1998).
Haschke, G., Schafer, H. & Diener, M. Effect of butyrate on membrane potential, ionic currents and intracellular Ca2+ concentration in cultured rat myenteric neurones. Neurogastroenterol. Motil. 14, 133–142 (2002).
Gola, M., Niel, J.P., Delmas, P. & Jacquet, G. Satellite glial cells in situ within mammalian prevertebral ganglia express K+ channels active at rest potential. J. Membr. Biol. 136, 75–84 (1993).
Delmas, P., Niel, J.P. & Gola, M. Muscarinic activation of a novel voltage-sensitive inward current in rabbit prevertebral sympathetic neurons. Eur. J. Neurosci. 8, 598–610 (1996).
Copel, C. et al. Activation of neurokinin 3 receptor increases Na(v)1.9 current in enteric neurons. J. Physiol. 587, 1461–1479 (2009).
Wood, J.D. Neuropathophysiology of functional gastrointestinal disorders. World J. Gastroenterol. 13, 1313–1332 (2007).
Clerc, N., Furness, J.B., Bornstein, J.C. & Kunze, W.A. Correlation of electrophysiological and morphological characteristics of myenteric neurons of the duodenum in the guinea-pig. Neuroscience 82 899–914 (1998).
Nurgali, K., Furness, J.B. & Stebbing, M.J. Correlation of electrophysiology, shape and synaptic properties of myenteric AH neurons of the guinea pig distal colon. Auton. Neurosci. 103, 50–64 (2003).
Nurgali, K., Stebbing, M.J. & Furness, J.B. Correlation of electrophysiological and morphological characteristics of enteric neurons in the mouse colon. J. Comp. Neurol. 468, 112–124 (2004).
Furness, J.B., Robbins, H.L., Xiao, J., Stebbing, M.J. & Nurgali, K. Projections and chemistry of Dogiel type II neurons in the mouse colon. Cell Tissue Res. 317, 1–12 (2004).
Li, W.C., Soffe, S.R. & Roberts, A. A direct comparison of whole cell patch and sharp electrodes by simultaneous recording from single spinal neurons in frog tadpoles. J. Neurophysiol. 92, 380–386 (2004).
Kunze, W.A., Bornstein, J.C., Furness, J.B., Hendriks, R. & Stephenson, D.S. Charybdotoxin and iberiotoxin but not apamin abolish the slow after-hyperpolarization in myenteric plexus neurons. Pflugers Arch. 428, 300–306 (1994).
Smith, T.K., Burke, E.P. & Shuttleworth, C.W. Topographical and electrophysiological characteristics of highly excitable S neurones in the myenteric plexus of the guinea-pig ileum. J. Physiol. 517, 817–830 (1999).
Iyer, V. et al. Electrophysiology of guinea-pig myenteric neurons correlated with immunoreactivity for calcium binding proteins. J. Auton. Nerv. Syst. 22, 141–150 (1988).
Gola, M. & Niel, J.P. Electrical and integrative properties of rabbit sympathetic neurones re-evaluated by patch clamping non-dissociated cells. J. Physiol. 460, 327–349 (1993).
Davie, J.T. et al. Dendritic patch-clamp recording. Nat. Protoc. 1, 1235–1247 (2006).
Mortensen, M. & Smart, T.G. Single-channel recording of ligand-gated ion channels. Nat. Protoc. 2, 2826–2841 (2007).
Walz, W. (ed) Patch-clamp analysis: Advanced Techniques, Second Edition Neuromethods 38 (Humana Press, Totowa, NJ, 2007).
Cummins, T.R., Rush, A.M., Estacion, M., Dib-Hajj, S.D. & Waxman, S.G. Voltage-clamp and current-clamp recordings from mammalian DRG neurons. Nat. Protoc. 4, 1103–1112 (2009).
Coste, B., Osorio, N., Padilla, F., Crest, M. & Delmas, P. Gating and modulation of presumptive NaV1.9 channels in enteric and spinal sensory neurons. Mol. Cell. Neurosci. 26, 123–134 (2004).
Padilla, F. et al. Expression and localization of the Nav1.9 sodium channel in enteric neurons and in trigeminal sensory endings: implication for intestinal reflex function and orofacial pain. Mol. Cell. Neurosci. 35, 138–152 (2007).
Galligan, J.J., Tatsumi, H., Shen, K.Z., Surprenant, A. & North, R.A. Cation current activated by hyperpolarization (IH) in guinea pig enteric neurons. Am. J. Physiol. 259, G966–G972 (1990).
Wood, J.D. Electrical and synaptic behavior of enteric neurons. In: Handbook of Physiology (eds. Schultz, S.G., Wood, J.D., Ranner, B.B.) 465–516 (American Physiological Society, Bethesda, Maryland, 1989).
Linden, D.R., Sharkey, K.A. & Mawe, G.M. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J. Physiol. 547, 589–601 (2003).
Lomax, A.E., Mawe, G.M. & Sharkey, K.A. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J. Physiol. 564, 863–875 (2005).
Kunze, W. et al. The soma and neurites of primary afferent neurons in the guinea-pig intestine respond differently to deformation. J. Physiol. 526, 375–385 (2000).
Mao, Y., Wang, B. & Kunze, W. Characterization of myenteric sensory neurons in the mouse small intestine. J. Neurophysiol. 96, 998–1010 (2006).
Acknowledgements
This study was supported by the CNRS and by grants from the Agence Nationale de la Recherche, Fondation Schlumberger, ARCInca-2006, UPSA, IRME and Fondation pour la Recherche Médicale.
Author information
Authors and Affiliations
Contributions
N.O. and P.D. conceived, designed and performed the experiments. N.O. and P.D. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Osorio, N., Delmas, P. Patch clamp recording from enteric neurons in situ. Nat Protoc 6, 15–27 (2011). https://doi.org/10.1038/nprot.2010.172
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.172
This article is cited by
-
A tissue-like neurotransmitter sensor for the brain and gut
Nature (2022)
-
The Effect of Substrate Stiffness on Cardiomyocyte Action Potentials
Cell Biochemistry and Biophysics (2016)
-
Electrophysiological Characteristics of Enteric Neurons Isolated from the Immortomouse
Digestive Diseases and Sciences (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.