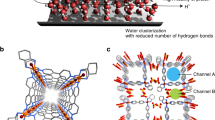
Abstract
It is known that at low temperature, water inside single-wall carbon nanotubes (water–SWNTs) undergoes a structural transition to form tube-like solid structures. The resulting ice NTs are hollow cylinders with diameters comparable to those of typical gas molecules. Hence, the gas-adsorption properties of ice– and water–SWNTs are of interest. Here, we carry out the first systematic investigation into the stability of water–SWNTs in various gas atmospheres below 0.1 MPa by means of electrical resistance, X-ray diffraction, NMR measurements and molecular dynamics calculations. It is found that the resistivity of water–SWNTs exhibits a significant increase in gas atmospheres below a critical temperature Tc, at which a particular type of atmospheric gas molecule enters the SWNTs in an on–off fashion. On the basis of this phenomenon, it is proposed that water–SWNTs can be used to fabricate a new type of molecular nanovalve.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Iijima, S. & Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 363, 603–615 (1993).
Bethune, D.S. et al. Cobalt-catalysed grown carbon nanotubes with single-atomic-layer walls. Nature 363, 605–607 (1993).
Smith, B. W., Monthioux, M. & Luzzi, D. E. Encapsulated C60 in carbon nanotubes. Nature 396, 323–324 (1998).
Fan, X. et al. Atomic arrangement of iodine atoms inside single-walled carbon nanotubes. Phys. Rev. Lett. 84, 4621 (2000).
Calbi, M. M., Cole, M. W., Gatica, S. M., Bojan, M. J. & Stan, G. Condensed phases of gases inside nanotube bundles. Rev. Mod. Phys. 73, 857–865 (2001).
Dillon, A. C. et al. Storage of hydrogen in single-walled carbon nanotubes. Nature 386, 377–379 (1997).
Hummer, G., Rasaiah, J. C. & Noworyta, J. P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414, 188–190 (2001).
Sanson, M. S. P. & Biggin, P. C. Water at the nanoscale. Nature 414, 156–157 (2001).
Mann, D. J. & Halls, M. D. Water alignment and proton conduction inside carbon nanotubes. Phys. Rev. Lett. 90, 195503 (2003).
Supple, S. & Quirke, N. Rapid imbibition of fluids in carbon nanotubes. Phys. Rev. Lett. 90, 214501 (2003).
Liu, Y. & Wang, Q. Transport behavior of water confined in carbon nanotubes. Phys. Rev. B 72, 085420 (2005).
Skoulidas, A. I., Ackerman, D. M., Johnson, J. K. & Sholl, D. S. Rapid transport of gases in carbon nanotubes. Phys. Rev. Lett. 89, 185901 (2002).
Holt, J. K. et al. Fast mass transport through sub–2-nanometer carbon nanotubes. Science 312, 1034–1037 (2006).
Weber, S. E., Talapatra, S., Journet, C., Zambano, A. & Migone, A. D. Determination of the binding energy of methane on single-walled carbon nanotube bundles. Phys. Rev. B 61, 13150 (2000).
Fujiwara, A. et al. Gas adsorption in the inside and outside of single-walled carbon nanotubes. Chem. Phys. Lett. 336, 205–211 (2001).
Kleinhammes, A. et al. Gas adsorption in single-walled carbon nanotubes studied by NMR. Phys. Rev. B 68, 075418 (2003).
Rols, S. et al. Argon adsorption in open-ended single-wall carbon nanotubes. Phys. Rev. B 71, 155411 (2005).
Maniwa, Y. et al. Anomaly of X-ray diffraction profile in single-walled carbon nanotubes. Japan. J. Appl. Phys. 38, L668–L670 (1999).
Maniwa, Y. et al. Phase transition in confined water inside carbon nanotubes. J. Phys. Soc. Jpn 71, 2863–2866 (2002).
Maniwa, Y. et al. Ordered water inside carbon nanotubes: Formation of pentagonal to octagonal ice-nanotubes. Chem. Phys. Lett. 401, 534–538 (2005).
Koga, K., Parra, R. D., Tanaka, H. & Zeng, X. C. Ice nanotube: What does the unit cell look like? J. Chem. Phys. 113, 5037–5040 (2000).
Koga, K., Gao, G. T., Tanaka, H. & Zeng, X. C. Formation of ordered ice nanotubes inside carbon nanotubes. Nature 412, 802–805 (2001).
Bai, J. et al. Ab initio studies of quasi-one-dimensional pentagon and hexagon ice nanotubes. J. Chem. Phys. 118, 3913–3916 (2003).
Byl, O. et al. Unusual hydrogen bonding in water-filled carbon nanotubes. J. Am. Chem. Soc. 128, 12090–12097 (2006).
Noon, W. H., Ausman, K. D., Smalley, R. E. & Ma, J. Helical ice-sheets inside carbon nanotubes in the physiological condition. Chem. Phys. Lett. 355, 445–448 (2002).
Tanaka, H. & Koga, K. Formation of ice nanotube with hydrophobic guests inside carbon nanotube. J. Chem. Phys. 123, 094706 (2005).
Journet, C. et al. Large-scale production of single-walled carbon nanotubes by the electric-arc technique. Nature 388, 756–758 (1997).
Kataura, H. et al. Optical properties of fullerene and non-fullerene peapods. Appl. Phys. A 74, 349–354 (2002).
Maniwa, Y. et al. Gas storage in single-walled carbon nanotubes. Mol. Cryst. Liq. Cryst. 340, 671–676 (2000).
Zahab, A., Spina, L., Poncharal, P. & Marliere, C. Water-vapor effect on the electrical conductivity of a single-walled carbon nanotube mat. Phys. Rev. B 62, 10000–10003 (2000).
Lee, R. S., Kim, H. J., Fischer, J. E., Thess, A. & Smalley, R. E. Conductivity enhancement in single-walled carbon nanotube bundles doped with K and Br. Nature 388, 255–257 (1997).
Zhou, W. et al. Charge transfer and Fermi level shift in p-doped single-walled carbon nanotubes. Phys. Rev. B 71, 205423 (2005).
Pati, R., Zhang, Y., Nayak, S. K. & Ajayan, P. M. Effect of H2O adsorption on electron transport in a carbon nanotube. Appl. Phys. Lett. 81, 2638–2640 (2002).
Thess, A. et al. Crystalline ropes of metallic carbon nanotubes. Science 273, 483–487 (1996).
Kadowaki, H. et al. Rietveld analysis and maximum entropy method of powder diffraction for bundles of single-walled carbon nanotubes. J. Phys. Soc. Jpn 74, 2290–2295 (2005).
Kolesnikov, A. I. et al. Anomalously soft dynamics of water in a nanotube: A revelation of nanoscale confinement. Phys. Rev. Lett. 93, 035503 (2004).
Smith, M. R. Jr et al. Selective oxidation of single-walled carbon nanotubes using carbon dioxide. Carbon 41, 1221–1230 (2003).
Nguyen, T. D. et al. A reversible molecular valve. Proc. Natl Acad. Sci. USA 102, 10029–10034 (2005).
Maniwa, Y. et al. Thermal expansion of single-walled carbon nanotube (SWNT) bundles: X-ray diffraction studies. Phys. Rev. B 64, 241402(R) (2001).
Maniwa, Y. et al. C70 molecular stumbling inside single-walled carbon nanotubes. J. Phys. Soc. Jpn 72, 45–48 (2003).
Abe, M. et al. Structural transformation from single-wall to double-wall carbon nanotube bundles. Phys. Rev. B 68, 041405R (2003).
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research by the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Contributions
The main contribution of each author to the present work was as follows. K.M. contributed to the NMR and resisitivity measurements, S.O. to the resisitivity and XRD measurements, H. Kyakuno to MD calculations, T.H. to the NMR measurements, H. Kadowaki to the data analysis, S.S. and Y.A. to the sample preparation, H. Kataura to the high-purity sample preparation and characterization and Y.M. to the project planning and data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary information I, II and II; figures S1, S2, S3, S4 and S5 (PDF 1032 kb)
Rights and permissions
About this article
Cite this article
Maniwa, Y., Matsuda, K., Kyakuno, H. et al. Water-filled single-wall carbon nanotubes as molecular nanovalves. Nature Mater 6, 135–141 (2007). https://doi.org/10.1038/nmat1823
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1823
This article is cited by
-
Nanopore structure analysis of single wall carbon nanotube xerogels and cryogels
Adsorption (2021)
-
Neutron scattering observation of quasi-free rotations of water confined in carbon nanotubes
Scientific Reports (2017)
-
Rotational dynamics and dynamical transition of water inside hydrophobic pores of carbon nanotubes
Scientific Reports (2017)
-
Observation of extreme phase transition temperatures of water confined inside isolated carbon nanotubes
Nature Nanotechnology (2017)
-
Ultrafast proton transport in sub-1-nm diameter carbon nanotube porins
Nature Nanotechnology (2016)