Abstract
Molecular biology is expected to provide new tools and approaches to assess the prognosis of patients with bladder cancer, by providing information on the risks of tumor recurrence and progression from superficial bladder cancer to an invasive phenotype. Genetic and epigenetic alterations have been closely associated with bladder carcinogenesis and progression, although most of these are still under investigation in a preclinical setting. This article highlights current findings from molecular studies, and describes their potential application in molecular staging of bladder cancer.
Key Points
-
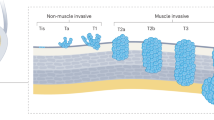
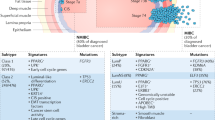
Activating mutations in oncogenes such as HRAS and in FGFR3 are reported in bladder cancer; in particular, a mutation of FGFR3 is evident in 70% of superficial bladder cancers of pTa stage
-
TP53 and RB1 genes are key regulators of the cell cycle, and mutations of TP53 are frequently found in high-grade, high-stage bladder cancers
-
Overexpression of p53 is thought to be a poor prognostic indicator in bladder cancer, although considerable variation in p53 expression is reported, depending on the assay conditions
-
Ki-67 antigen is expressed in the nucleus of proliferating cells and the labeling index of Ki-67 expression (measured by immunostaining using MIB-1 monoclonal antibody) is thought to be a prognostic marker for bladder cancer
-
Chromosome losses are available as markers of bladder cancer; loss of chromosome 9 is the most frequent genetic alteration in bladder cancer, and loss of 17p or the TP53 locus is observed in most invasive bladder cancers
-
Genome-wide DNA analysis, using gene chips or complementary DNA arrays, are powerful tools for data mining, but further statistical validation is required before these tools can have clinical applications in cancer staging
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
American Cancer Society (2005) Cancer Facts and Figures 2005. Atlanta: American Cancer Society
Japanese Urological Association (1997) The report of clinical statistical studies on registered bladder cancer patients in Japan. Japanese Urological Association, Tokyo
Kurth KH et al. (1995) Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer 31 (Pt A): 1840–1846
Knowles MA (2001) What we could do now: molecular pathology of bladder cancer. Mol Pathol 54: 215–221
Habuchi T et al. (2005) Prognostic markers for bladder cancer: international consensus panel on bladder tumor markers. Urology 66 (Suppl 1): 64–74
Wolff EM et al. (2005) Mechanisms of disease: genetic and epigenetic alterations that drive bladder cancer. Nat Clin Pract Urol 2: 502–510
Tabin CJ et al. (1982) Mechanism of activation of a human oncogene. Nature 300: 143–149
Reddy EP et al. (1982) A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature 300: 149–152
Taparowsky E et al. (1982) Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature 300: 762–765
Knowles MA et al. (1993) H-ras is infrequent in bladder cancer: confirmation by single-strand conformation polymorphism analysis, designed restriction fragment length polymorphisms, and direct sequencing. Cancer Res 53: 133–139
Sauter G et al. (1994) Epidermal-growth-factor-receptor expression is associated with rapid tumor proliferation in bladder cancer. Int J Cancer 57: 508–514
Kruger S et al. (2002) Overexpression of c-erbB-2 oncoprotein in muscle-invasive bladder carcinoma: relationship with gene amplification, clinicopathological parameters and prognostic outcome. Int J Oncol 21: 981–987
Underwood M et al. (1995) C-erbB-2 gene amplification: a molecular marker in recurrent bladder tumors? Cancer Res 55: 2422–2430
Horton WA and Hecht JT (2002) Skeletal dysplasias. In Connective Tissue and its Heritable Disorders, 901–908 (eds Royce PM and Steinmann B). New York: Wiley
Cappellen D et al. (1999) Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet 23: 18–20
Billerey C et al. (2001) Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol 158: 1955–1959
van Rhijn BW et al. (2002) Frequent FGFR3 mutations in urothelial papilloma. J Pathol 198: 245–251
Su WC et al. (1997) Activation of Stat1 by mutant fibroblast growth-factor receptor in thanatophoric dysplasia type II dwarfism. Nature 386: 288–292
Jebar AH et al. (2005) FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene 24: 5218–5225
Hernandez S et al. (2005) FGFR3 and TP53 mutations in T1G3 transitional bladder carcinomas: independent distribution and lack of association with prognosis. Clin Cancer Res 11: 5444–5450
Kimura T et al. (2001) The incidence of thanatophoric dysplasia mutations in the FGFR3 gene is higher in low-grade or superficial bladder carcinomas. Cancer 92: 2555–2561
van Rhijn BW et al. (2001) The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Res 61: 1265–1268
van Rhijn BW et al. (2003) Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J Clin Oncol 21: 1912–1921
Zieger K et al. (2005) Role of activating fibroblast growth factor receptor 3 mutations in the development of bladder tumors. Clin Cancer Res 11: 7709–7719
Olumi AF et al. (1990) Allelic loss of chromosome 17p distinguishes high grade from low grade transitional cell carcinomas of the bladder. Cancer Res 50: 7081–7083
Sidransky D et al. (1991) Identification of p53 gene mutations in bladder cancers and urine samples. Science 252: 706–709
Fujimoto K et al. (1992) Frequent association of p53 gene mutation in invasive bladder cancer. Cancer Res 52: 1393–1398
Sarkis AS et al. (1993) Nuclear overexpression of p53 protein in transitional cell bladder carcinoma: a marker for disease progression. J Natl Cancer Inst 85: 53–59
Esrig D et al. (1994) Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med 331: 1259–1264
Schmitz-Drager BJ et al. (2000) P53 immunohistochemistry as a prognostic marker in bladder cancer. Playground for urology scientists? Eur Urol 38: 691–699
McShane LM et al. (2000) Reproducibility of p53 immunohistochemistry in bladder tumors. National Cancer Institute, Bladder Tumor Marker Network. Clin Cancer Res 6: 1854–1864
Proctor AJ et al. (1991) Amplification at chromosome 11q13 in transitional cell tumours of the bladder. Oncogene 6: 789–795
Cairns P et al. (1991) Loss of heterozygosity at the RB locus is frequent and correlates with muscle invasion in bladder carcinoma. Oncogene 6: 2305–2309
Miyamoto H et al. (1995) Retinoblastoma gene mutations in primary human bladder cancer. Br J Cancer 71: 831–835
Lianes P et al. (1994) Altered patterns of MDM2 and TP53 expression in human bladder cancer. J Natl Cancer Inst 86: 1325–1330
Cordon-Cardo C et al. (1992) Altered expression of the retinoblastoma gene product: prognostic indicator in bladder cancer. J Natl Cancer Inst 84: 1251–1256
Logothetis CJ et al. (1992) Altered expression of retinoblastoma protein and known prognostic variables in locally advanced bladder cancer. J Natl Cancer Inst 84: 1256–1261
Cote RJ et al. (1998) Elevated and absent pRb expression is associated with bladder cancer progression and has cooperative effects with p53. Cancer Res 58: 1090–1094
Chatterjee SJ et al. (2004) Hyperphosphorylation of pRb: a mechanism for RB tumour suppressor pathway inactivation in bladder cancer. J Pathol 203: 762–770
Chatterjee SJ et al. (2004) Combined effects of p53, p21, and pRb expression in the progression of bladder transitional cell carcinoma. J Clin Oncol 22: 1007–1013
Cina SJ et al. (2001) Correlation of Ki-67 and p53 with the New World Health Organization/International Society of Urological Pathology classification system for urothelial neoplasia. Arch Pathol Lab Med 125: 646–651
Dalbagni G et al. (1993) Genetic alterations in bladder cancer. Lancet 342: 469–471
Knowles MA et al. (1994) Allelotype of human bladder cancer. Cancer Res 54: 531–538
Shigyo M et al. (1998) Allelic loss on chromosome 9 in bladder cancer tissues and urine samples detected by blunt-end single-strand DNA conformation polymorphism. Int J Cancer 78: 425–429
Williams SV et al. (2002) Molecular genetic analysis of chromosome 9 candidate tumor-suppressor loci in bladder cancer cell lines. Genes Chromosomes Cancer 34: 86–96
Emmott RC et al. (1979) Correlation of the cell surface antigens with stage and grade in cancer of the bladder. J Urol 121: 37–39
Richie JP et al. (1980) Immunologic indicators of prognosis in bladder cancer: the importance of cell surface antigens. J Urol 123: 22–24
Chihara Y et al. (2005) Loss of blood group A antigen expression in bladder cancer caused by allelic loss and/or methylation of the ABO gene. Lab Invest 85: 895–907
Sugano K et al. (1997) Diagnosis of bladder cancer by analysis of the allelic loss of the p53 gene in urine samples using blunt-end single-strand conformation polymorphism. Int J Cancer 74: 403–406
Shigyo M et al. (2001) Molecular follow-up of newly diagnosed bladder cancer using urine samples. J Urol 166: 1280–1285
Jones PA and Laird PW (1999) Cancer epigenetics comes of age. Nat Genet 21: 163–167
Maruyama R et al. (2001) Aberrant promoter methylation profile of bladder cancer and its relationship to clinicopathological features. Cancer Res 61: 8659–8563
Catto JW et al. (2005) Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J Clin Oncol 23: 2903–2910
Kawamoto K et al. (2006) p16INK4a and p14ARF methylation as a potential biomarker for human bladder cancer. Biochem Biophys Res Commun 339: 790–796
Habuchi T et al. (1998) Structure and methylation-based silencing of a gene (DBCCR1) within a candidate bladder cancer tumor suppressor region at 9q32–q33. Genomics 48: 277–288
Tada Y et al. (2002) The association of death-associated protein kinase hypermethylation with early recurrence in superficial bladder cancers. Cancer Res 62: 4048–4053
Bornman DM et al. (2001) Methylation of the E-cadherin gene in bladder neoplasia and in normal urothelial epithelium from elderly individuals. Am J Pathol 159: 831–835
Horikawa Y et al. (2003) Hypermethylation of an E-cadherin (CDH1) promoter region in high grade transitional cell carcinoma of the bladder comprising carcinoma in situ. J Urol 169: 1541–1545
Uzzo RG et al. (2004) Detection of bladder cancer in urine by a tumor suppressor gene hypermethylation panel. Clin Cancer Res 10: 1887–1893
Sen S et al. (2002) Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst 94: 1320–1329
Meraldi P et al. (2002) Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J 21: 483–492
Yamamoto Y et al. (2004) Centrosome hyperamplification predicts progression and tumor recurrence in bladder cancer. Clin Cancer Res 10: 6449–6455
Primdahl H et al. (2002) Allelic imbalances in human bladder cancer: genome-wide detection with high-density single-nucleotide polymorphism arrays. J Natl Cancer Inst 94: 216–223
Blaveri E et al. (2005) Bladder cancer stage and outcome by array-based comparative genomic hybridization. Clin Cancer Res 11: 7012–7022
Modlich O et al. (2004) Identifying superficial, muscle-invasive, and metastasizing transitional cell carcinoma of the bladder: use of cDNA array analysis of gene expression profiles. Clin Cancer Res 10: 3410–3421
Sanchez-Carbayo M et al. (2006) Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol 24: 778–789
Takata R et al. (2005) Predicting response to methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy for bladder cancers through genome-wide gene expression profiling. Clin Cancer Res 11: 2625–2636
Sanchez-Carbayo M et al. (2006) Profiling bladder cancer using targeted antibody arrays. Am J Pathol 168: 93–103
Ludwig JA et al. (2005) Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer 5: 845–856
Trudel S et al. (2004) Inhibition of fibroblast growth factor receptor 3 induces differentiation and apoptosis in t(4;14) myeloma. Blood 103: 3521–3528
Paterson JL et al. (2004) Preclinical studies of fibroblast growth factor receptor 3 as a therapeutic target in multiple myeloma. Br J Haematol 124: 595–603
Cheng JC et al. (2004) Continuous zebularine treatment effectively sustains demethylation in human bladder cancer cells. Mol Cell Biol 24: 1270–1278
Kuball J et al. (2002) Successful adenovirus-mediated wild-type p53 gene transfer in patients with bladder cancer by intravesical vector instillation. J Clin Oncol 20: 957–965
Pagliaro LC et al. (2003) Repeated intravesical instillations of an adenoviral vector in patients with locally advanced bladder cancer: a phase I study of p53 gene therapy. J Clin Oncol 21: 2247–2253
Acknowledgements
We thank Dr Yoshitomo Chihara for his cooperation in preparing experimental data. This work was supported in part by Grants-in-Aid for Cancer Research, and for the Third Term Comprehensive 10-year Program for Cancer Control from the Ministry of Health, Labour and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sugano, K., Kakizoe, T. Genetic alterations in bladder cancer and their clinical applications in molecular tumor staging. Nat Rev Urol 3, 642–652 (2006). https://doi.org/10.1038/ncpuro0649
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro0649
This article is cited by
-
Diagnostic markers of urothelial cancer based on DNA methylation analysis
BMC Cancer (2013)
-
Clinical significance of subepithelial growth patterns in non-muscle invasive bladder cancer
BMC Urology (2011)
-
The use of Urovysion™ fluorescence in situ hybridization in the diagnosis and surveillance of non-urothelial carcinoma of the bladder
Modern Pathology (2009)