Abstract
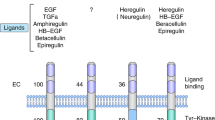
The human epidermal growth factor receptor-2 (HER2) is overexpressed and/or amplified in up to 25% of breast cancer patients, and this feature is associated with an aggressive phenotype, high recurrence rate and reduced survival. Until recently, combination chemotherapy (with or without endocrine therapy) was the only effective adjuvant treatment for HER2-positive patients. Trastuzumab is a monoclonal antibody directed against the HER2 extracellular domain, and five recent adjuvant breast cancer trials have demonstrated an astonishing and highly reproducible benefit in halving the recurrence rate and reducing mortality in patients with this phenotype. Many questions related to trastuzumab use in the adjuvant setting still remain; these include the optimum timing and duration of treatment, trastuzumab use with taxanes and radiotherapy, its role in small node-negative tumors, the optimum chemotherapy regimens and cost-effectiveness. This Review outlines the five adjuvant trastuzumab studies and discusses the controversies and challenges that have emerged for both the clinician and healthcare authorities worldwide as a consequence of the results from these trials.
Key Points
-
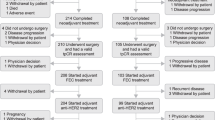
Five adjuvant trials with trastuzumab have shown clinical benefit in reducing recurrence rate and improving overall survival; however, many controversies remain regarding adjuvant trastuzumab use owing to the varying trial designs and patient populations
-
The optimum timing of trastuzumab initiation is unclear, although delayed therapy does not seem to be less efficacious and combined therapy (with taxanes with and without radiotherapy) is no more toxic
-
The empirical standard for trastuzumab therapy is 12 months, although there is evidence from the FinHer trial for a shorter duration of treatment
-
Results from the NCCTG-N9831 trial show that concurrent administration with taxanes may be more efficacious, but more cardiac events were also noted
-
Endocrine responsiveness may be a useful tool to further stratify HER2-positive, node-negative patients with tumors smaller than 1 cm
-
Optimum chemotherapy regimens have not been defined, but early evidence for non-anthracycline regimens is promising
-
Trastuzumab is cost-effective but still not affordable for many countries
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Slamon DJ et al. (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235: 177–182
Slamon DJ et al. (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707–712
Slamon DJ et al. (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792
Marty M et al. (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23: 4265–4274
Piccart-Gebhart MJ et al. (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353: 1659–1672
Smith I et al. (2007) 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER-2 positive breast cancer: a randomized controlled trial. Lancet 369: 29–36
Romond EH et al. (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353: 1673–1684
Perez EA et al. (2005) NCCTG-N9831. May 2005 Update. Presented at the 41st American Society of Clinical Oncology Annual Meeting, 2005 May 13–17, Orlando, FL [http://www.asco.org/ac/1,10003,_12-002511-00_18-0034 -00_19-005815-00_21-001,00.asp]
Perez EA et al. (2007) Updated results of the combined analysis of NCCTG N9831 and NSABP B-31 adjuvant chemotherapy with / without trastuzumab in patients with HER2-positive breast cancer. J Clin Oncol 25 (18 Suppl): LBA512
Slamon D et al. (2005) Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study [abstract #1]. Breast Cancer Res Treat 94 (Suppl 1): S5
Slamon D et al. (2006) BCIRG 006: 2nd interim analysis phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC-T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC-TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients. Breast Cancer Res Treat 100: S2
Joensuu H et al. (2006) Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 354: 809–820
Baselga J et al. (1999) Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 59: 2825–2831
Pegram M et al. (1999) Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 18: 2241–2251
Seidman A et al. (2002) Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20: 1215–1221
Rastogi P et al. (2007) Five year update of cardiac dysfunction on NSABP B-31, a randomized trial of sequential doxorubicin/cyclophosphamide (AC)→paclitaxel (T) vs AC→T with trastuzumab (H). J Clin Oncol 25 (18 Suppl): LBA512
Suter TM et al. (2007) Trastuzumab-associated cardiac adverse effects in the Herceptin Adjuvant trial. J Clin Oncol 25: 1–8
Pietras RJ et al. (1999) Monoclonal antibody to HER-2/neu receptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res 59: 1347–1355
Halyard MY et al. (2006) Adjuvant radiotherapy and trastuzumab in stage I-IIA breast cancer: Toxicity data from North Central Cancer Treatment Group Phase III trial N9831. J Clin Oncol 24 (18 Suppl): LBA523
Leyland-Jones B et al. (2003) Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol 21: 3965–3971
Early Breast Cancer Trialists' Collaborative Group: (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 265: 1687–1717
Goldhirsh A et al. (2007) Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 18: 1133–1144
Gelber RD et al. (2003) Tailoring adjuvant treatments for the individual breast cancer patient. The Breast 12: 558–568
Johnston SR et al. (2007) Clinical strategies for rationale combinations of aromatase inhibitors with novel therapies for breast cancer. J Steroid Biochem Mol Biol 106: 180–186
Osborne CK et al. (2003) Role of estrogen receptor coactivator AIB1 (SRC3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95: 353–361
Mackey JR et al. (2006) Trastuzumab prolongs progression-free survival in hormone-dependent and HER-2 positive metastatic breast cancer. Presented at the San Antonio Breast Cancer Symposium. 2006 December 14–17 [http://www.abstracts2view.com/sabcs06/view.php?nu=SABCS06L_665]
Durbecq V et al. (2004) Correlation between topoisomerase II-alpha gene amplification and protein expression in HER-2 amplified breast cancer. Int J Oncol 25: 1473–1479
Durbecq V et al. (2004) Topoisomerase-II alpha expression as a predictive marker in a population of advanced breast cancer patients randomly treated either with single-agent doxorubicin or single-agent docetaxel. Mol Cancer Ther 3: 1207–1214
Cardoso F et al. (2004) Correlation between complete response to anthracycline-based chemotherapy and topoisomerase-II gene amplification and protein overexpression in locally advanced/metastatic breast cancer. Int J Oncol 24: 201–209
Park K et al. (2006) Topoisomerase II-alpha gene deletion is not as frequent as its amplification in breast cancer. Breast Cancer Res Treat 98: 337–342
Tanner M et al. (2006) Topoisomerase II-alpha gene amplification predicts favorable treatment response to tailored and dose-escalated anthracycline-based adjuvant chemotherapy in HER-2/neu-amplified breast cancer: Scandinavian Breast Group Trial 9401. J Clin Oncol 24: 2409–2411
Knoop AS et al. (2005) Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophoshamide, epirubicin, and fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol 23: 7483–7490
Coon JS et al. (2002) Amplification and overexpression of topoisomerase II-alpha predict response to anthracycline-based therapy in locally advanced breast cancer. Clin Cancer Res 8: 1061–1067
Di Leo A et al. (2002) HER-2 amplification and topoisomerase II-alpha gene aberrations as predictive markers in node-positive breast cancer patients randomly treated either with an anthracycline-based therapy or with cyclophosphamide, methotrexate, and 5-fluorouracil. Clin Cancer Res 8: 1107–1116
Park K et al. (2003) Topoisomerase II-alpha (topoII) and HER-2 amplification in breast cancers and response to preoperative doxorubicin chemotherapy. Eur J Cancer 39: 631–634
Jarvinen TA and Liu ET (2003) Her-2/neu and topoisomerase II alpha in breast cancer. Breast Cancer Res 78: 299–311
Withoff S et al. (1996) Selection of a subpopulation with fever DNA topoisomerase II α gene copies in a doxorubicin-resistant cell line panel. Br J Cancer 74: 502–507
Jarvinen TA et al. (1999) Characterisation of topoisomerase IIα gene amplification and deletion in breast cancer. Genes Chromosomes Cancer 26: 142–150
Gopalakrishnan S and Linnane J (2005) Adjuvant trastuzumab for breast cancer: the other side of the coin. BMJ 331: 1202–1203
Imai H et al. (2007) Economic evaluation of the prevention and treatment of breast cancer—present status and open issues. Breast Cancer 14: 81–87
Liberato NL et al. (2007) Cost effectiveness of adjuvant trastuzumab in human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 25: 625–633
Shiroiwa T et al. (2007) The model-based cost-effectiveness analysis of 1-year adjuvant trastuzumab treatment: based on 2-year follow-up HERA trial data. Breast Cancer Res Treat 109: 559–566
Neyt M et al. (2006) An economic evaluation of Herceptin in adjuvant setting: the Breast Cancer International Research Group 006 trial. Ann Oncol 17: 381–390
Millar JA and Millward MJ (2007) Cost effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: a lifetime model. Pharmacoeconomics 25: 429–442
Nagy P et al. (2005) Decreased accessibility and lack of activation or ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res 65: 473–482
Nagata Y et al. (2004) PTEN activation contributes to tumor inhibition by trastuzumab and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6: 117–127
Nahta R et al. (2004) p27(kip1) downregulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res 64: 3981–3986
Lu YH et al. (2001) Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Nat Cancer Inst 93: 1852–1857
Nahta R et al. (2005) Insulin-like growth factor-I receptor / human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 65: 11118–11128
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
MJ Piccart-Gebhart is a consultant for Boehringer Ingelheim and GlaxoSmithKline, and receives grant/research support from Boehringer Ingelheim, GlaxoSmithKline and Roche. The other authors declared no competing interests.
Rights and permissions
About this article
Cite this article
Dinh, P., de Azambuja, E., Cardoso, F. et al. Facts and controversies in the use of trastuzumab in the adjuvant setting. Nat Rev Clin Oncol 5, 645–654 (2008). https://doi.org/10.1038/ncponc1219
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncponc1219