Abstract
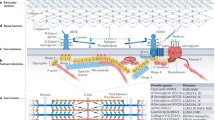
Recent studies have defined a group of muscular dystrophies, now termed the dystroglycanopathies, as novel disorders of glycosylation. These conditions include Walker–Warburg syndrome, muscle–eye–brain disease, Fukuyama-type congenital muscular dystrophy, congenital muscular dystrophy types 1C and 1D, and limb-girdle muscular dystrophy type 2I. Although clinical findings can be highly variable, dystroglycanopathies are all characterized by cortical malformations and ocular defects at the more severe end of the clinical spectrum, in addition to muscular dystrophy. All of these disorders are defined by the underglycosylation of α-dystroglycan. Defective glycosylation of dystroglycan severs the link between this important cell adhesion molecule and the extracellular matrix, thereby contributing to cellular pathology. Recent experiments indicate that glycosylation might not only define forms of muscular dystrophy but also provide an avenue to the development of therapies for these disorders.
Key Points
-
Mutations in 12 different genes have been shown to cause forms of congenital muscular dystrophy
-
Six of the genes associated with congenital muscular dystrophy code for molecules that are involved in the glycosylation of α-dystroglycan, a membrane protein that binds to the extracellular matrix
-
Dystroglycanopathies—diseases resulting from mutations that affect α-dystroglycan glycosylation—include Walker–Warburg syndrome, muscle–eye–brain disease, Fukuyama-type congenital muscular dystrophy, congenital muscular dystrophy types 1C and 1D, and limb-girdle muscular dystrophy type 2I
-
α-Dystroglycan is roughly half carbohydrate by molecular weight, which is largely attributable to the presence of a serine–threonine-rich domain that contains up to 55 sites for O-linked glycosylation
-
All dystroglycanopathies have the common molecular finding that α-dystroglycan is underglycosylated, and most dystroglycanopathy-associated gene products are thought to be involved in the synthesis of O-linked mannose chains on α-dystroglycan
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jimenez-Mallebrera C et al. (2005) Congenital muscular dystrophy: molecular and cellular aspects. Cell Mol Life Sci 62: 809–823
van Reeuwijk J et al. (2005) Glyc-O-genetics of Walker–Warburg syndrome. Clin Genet 67: 281–289
Martin PT and Freeze HH (2003) Glycobiology of neuromuscular disorders. Glycobiology 13: 67R–75R
Muntoni F et al. (2002) Defective glycosylation in muscular dystrophy. Lancet 360: 1419–1421
Michele DE and Campbell KP (2003) Dystrophin–glycoprotein complex: post-translational processing and dystroglycan function. J Biol Chem 278: 15457–15460
Endo T (2004) Structure, function and pathology of O-mannosyl glycans. Glycoconjugate J 21: 3–7
Schachter H et al. (2004) The role of defective glycosylation in congenital muscular dystrophy. Glycoconjugate J 20: 291–300
Endo T and Toda T (2003) Glycosylation and congenital muscular dystrophies. Biol Pharm Bull 26: 1641–1647
Michele DE et al. (2002) Post-translational disruption of dystroglycan–ligand interactions in congenital muscular dystrophies. Nature 418: 417–422
Beltran-Valero de Bernabe D et al. (2002) Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker–Warburg syndrome. Am J Hum Genet 71: 1033–1043
van Reeuwijk J et al. (2005) POMT2 mutations cause alpha-dystroglycan hypoglycosylation and Walker–Warburg syndrome. J Med Genet 42: 907–912
Yoshida A et al. (2001) Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell 1: 717–724
Kobayashi K et al. (1998) An ancient retrotransposonal insertion causes Fukuyama-type congenital muscular dystrophy. Nature 394: 388–392
Brockington M et al. (2001) Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin α2 deficiency and abnormal glycosylation of α-dystroglycan. Am J Hum Genet 69: 1198–1209
Longman C et al. (2003) Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of α-dystroglycan. Hum Mol Genet 12: 2853–2861
Brockington M et al. (2001) Mutations in the fukutin-related protein gene (FKRP) identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum Mol Genet 10: 2851–2859
Qu Q and Smith FI (2004) Alpha-dystroglycan interactions affect cerebellar granule neuron migration. J Neurosci Res 76: 771–782
Qu Q and Smith FI (2005) Neuronal migration defects in cerebellum of the Largemyd mouse are associated with disruptions in Bergman glia organization and delayed migration of granule neurons. Cerebellum 4: 261–270
Montanaro F and Carbonetto S (2003) Targeting dystroglycan in the brain. Neuron 37: 193–196
Topaloglu H et al. (2003) FKRP gene mutations cause congenital muscular dystrophy, mental retardation, and cerebellar cysts. Neurology 60: 988–992
Louhichi N et al. (2004) New FKRP mutations causing congenital muscular dystrophy associated with mental retardation and central nervous system abnormalities: identification of a founder mutation in Tunisian families. Neurogenetics 5: 27–34
Beltran-Valero de Bernabe D et al. (2004) Mutations in the FKRP gene can cause muscle–eye–brain disease and Walker–Warburg syndrome. J Med Gen 41: e61
De Paula F et al. (2003) Asymptomatic carriers for homozygous novel mutations in the FKRP gene: the other end of the spectrum. Eur J Hum Genet 11: 923–930
Kondo-Iida E et al. (1999) Novel mutations and genotype–phenotype relationships in 107 families with Fukuyama-type congenital muscular dystrophy (FCMD). Hum Mol Genet 8: 2303–2309
Beltran–Valero de Bernabe D et al. (2003) A homozygous nonsense mutation in the Fukutin gene causes a Walker–Warburg syndrome phenotype. J Med Genet 40: 835–838
Balci B et al. (2005) An autosomal recessive limb girdle muscular dystrophy (LGMD2) with mild mental retardation is allelic to Walker–Warburg syndrome (WWS) caused by a mutation in the POMT1 gene. Neuromuscul Disord 15: 271–275
Taniguchi K et al. (2003) Worldwide distribution and broader clinical spectrum of muscle–eye–brain disease. Hum Mol Genet 12: 527–534
Haliloglu G et al. (2004) Clinical spectrum of muscle–eye–brain disease: from the typical presentation to severe autistic features. Acta Myol 23: 137–139
Ervasti JM and Campbell KP (1991) Membrane organization of the dystrophin–glycoprotein complex. Cell 66: 1121–1131
Ibraghimov-Beskrovnaya O et al. (1992) Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355: 696–702
Martin PT (2003) Dystroglycan glycosylation and its role in matrix binding in skeletal muscle. Glycobiology 13: 55R–66R
Blake DJ et al. (2002) Function and genetics of dystrophin and dystrophin-associated proteins in muscle. Physiol Rev 82: 291–329
Mathews KD and Moore SA (2003) Limb-girdle muscular dystrophy. Curr Neurol Neurosci Rep 3: 78–85
Chiba A et al. (1997) Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve α-dystroglycan. J Biol Chem 272: 2156–2162
Sasaki T et al. (1998) Detection of O-mannosyl glycans in rabbit skeletal muscle α-dystroglycan. Biochem Biophys Acta 1425: 599–606
Smalheiser NR et al. (1998) Structural analysis of sequences O-linked to mannose reveals a novel Lewis X structure in cranin (dystroglycan) purified from sheep brain. J Biol Chem 273: 23689–23703
Willer T et al. (2003) O-mannosyl glycans: from yeast to novel associations with human disease. Curr Opin Stru Biol 13: 621–630
Moore SA et al. (2002) Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature 418: 422–425
Saito F et al. (2003) Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron 38: 747–758
Cohn RD et al. (2002) Disruption of dag1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell 110: 639–648
Martin PT et al. (1999) Distinct structures and functions of related pre- and postsynaptic carbohydrates at the mammalian neuromuscular junction. Mol Cell Neurosci 13: 105–118
Akasaka-Manya K et al. (2003) Mutations in the POMT1 gene found in patients with Walker–Warburg syndrome lead to a defect in protein O-mannosylation. Biochem Biophys Res Commun 325: 75–79
Ichimiya T et al. (2004) The twisted abdomen phenotype of Drosophila POMT1 and POMT2 mutants coincides with their heterophilic protein O-mannosyltransferase activity. J Biol Chem 279: 42638–42647
Manya H et al. (2004) Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity. Proc Natl Acad Sci USA 101: 500–505
Willer T et al. (2004) Targeted disruption of the Walker–Warburg syndrome gene Pomt1 in mouse results in embryonic lethality. Proc Natl Acad Sci USA 101: 14126–14131
Williamson RA et al. (1997) Dystroglycan is essential for early embryonic development: disruption of Reichert's membrane in Dag-1 null mice. Hum Mol Genet 6: 831–841
Jayasinha V et al. (2003) Inhibition of dystroglycan cleavage causes muscular dystrophy in transgenic mice. Neuromuscul Disord 13: 365–375
Manya H et al. (2003) Loss-of-function of an N-acetylglucosaminyltransferase, POMGnT1, in muscle–eye–brain disease. Biochem Biophys Res Commun 306: 93–97
Zhang W et al. (2003) Enzymatic diagnostic test for muscle–eye–brain type congenital muscular dystrophy using commercially available reagents. Clin Biochem 36: 339–344
Vajsar J et al. (2006) Carriers and patients with muscle–eye–brain disease can be rapidly diagnosed by enzymatic analysis of fibroblasts and lymphoblasts. Neuromuscul Disord [doi:10.1016/j.nmd.2005.11.012]
Hayashi YK et al. (2001) Selective deficiency of α-dystroglycan in Fukuyama-type congenital muscular dystrophy. Neurology 57: 115–121
Matsumoto H et al. (2004) Subcellular localization of fukutin and fukutin-related protein in muscle cells. J Biochem 135: 709–712
Takeda S et al. (2003) Fukutin is required for maintenance of muscle integrity, cortical histiogenesis and normal eye development. Hum Mol Genet 12: 1449–1459
Torelli S et al. (2005) Sub-cellular localisation of fukutin related protein in different cell lines and in the muscle of patients with MDC1C and LGMD2I. Neuromuscul Disord 15: 836–843
Brown SC et al. (2004) Abnormalities in α-dystroglycan expression in MDC1C and LGMD2I muscular dystrophies. Am J Pathol 164: 727–737
Grewal PK et al. (2001) Mutant glycosyltransferase and altered glycosylation of α-dystroglycan in the myodystrophy mouse. Nature Genet 28: 151–154
Barresi R et al. (2004) LARGE can functionally bypass α-dystroglycan glycoyslation defects in distinct congenital muscular dystrophies. Nature Med 10: 696–703
Esko JD and Selleck SB (2002) Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem 71: 435–471
Patnaik SK and Stanley P (2005) Mouse large can modify complex N- and mucin O-glycans on α-dystroglycan to induce laminin binding. J Biol Chem 280: 20851–20859
Combs AC and Ervasti JM (2005) Enhanced laminin binding by α-dystroglycan after enzymatic digestion. Biochem J 390: 303–309
Fujimura K et al. (2005) LARGE2 facilitates the maturation of α-dystroglycan more effectively than LARGE. Biochem Biophys Res Commun 329: 1162–1171
Grewal PK et al. (2005) Characterization of the LARGE family of putative glycosyltransferases associated with dystroglycanopathies. Glycobiology 15: 912–923
Brockington M et al. (2005) Localization and functional analysis of the LARGE family of glycosyltransferases: significance for muscular dystrophy. Hum Mol Genet 14: 657–665
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Martin, P. Mechanisms of Disease: congenital muscular dystrophies—glycosylation takes center stage. Nat Rev Neurol 2, 222–230 (2006). https://doi.org/10.1038/ncpneuro0155
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0155
This article is cited by
-
The role of protein glycosylation in muscle diseases
Molecular Biology Reports (2022)
-
NAD+ improves neuromuscular development in a zebrafish model of FKRP-associated dystroglycanopathy
Skeletal Muscle (2019)
-
Implications for the mammalian sialidases in the physiopathology of skeletal muscle
Skeletal Muscle (2012)
-
Congenital muscular dystrophy type 1D (MDC1D) due to a large intragenic insertion/deletion, involving intron 10 of the LARGE gene
European Journal of Human Genetics (2011)
-
Novel synonymous substitution in POMGNT1 promotes exon skipping in a patient with congenital muscular dystrophy
Journal of Human Genetics (2008)