Abstract
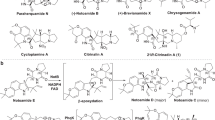
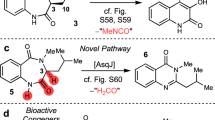
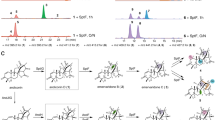
Spirotryprostatins, an indole alkaloid class of nonribosomal peptides isolated from Aspergillus fumigatus, are known for their antimitotic activity in tumor cells. Because spirotryprostatins and many other chemically complex spiro-carbon–bearing natural products exhibit useful biological activities, identifying and understanding the mechanism of spiro-carbon biosynthesis is of great interest. Here we report a detailed study of spiro-ring formation in spirotryprostatins from tryprostatins derived from the fumitremorgin biosynthetic pathway, using reactants and products prepared with engineered yeast and fungal strains. Unexpectedly, FqzB, an FAD-dependent monooxygenase from the unrelated fumiquinazoline biosynthetic pathway, catalyzed spiro-carbon formation in spirotryprostatin A via an epoxidation route. Furthermore, FtmG, a cytochrome P450 from the fumitremorgin biosynthetic pathway, was determined to catalyze the spiro-ring formation in spirotryprostatin B. Our results highlight the versatile role of oxygenating enzymes in the biosynthesis of structurally complex natural products and indicate that cross-talk of different biosynthetic pathways allows product diversification in natural product biosynthesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cui, C.-B., Kakeya, H. & Osada, H. Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 52, 12651–12666 (1996).
Cui, C.-B., Kakeya, H. & Osada, H. Spirotryprostatin B, a novel mammalian cell cycle inhibitor produced by Aspergillus fumigatus. J. Antibiot. (Tokyo) 49, 832–835 (1996).
Allen, J.D. et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol. Cancer Ther. 1, 417–425 (2002).
Edmondson, S.D. & Danishefsky, S.J. The total synthesis of spirotryprostatin A. Angew. Chem. Int. Edn Engl. 37, 1138–1140 (1998).
Edmondson, S., Danishefsky, S.J., Sepp-Lorenzino, L. & Rosen, N. Total synthesis of spirotryprostatin A, leading to the discovery of some biologically promising analogues. J. Am. Chem. Soc. 121, 2147–2155 (1999).
Wang, H. & Ganesan, A. A biomimetic total synthesis of (−)-spirotryprostatin B and related studies. J. Org. Chem. 65, 4685–4693 (2000).
Williams, R.M. Total synthesis and biosynthesis of the paraherquamides: an intriguing story of the biological Diels–Alder construction. Chem. Pharm. Bull. (Tokyo) 50, 711–740 (2002).
Li, S.M. Genome mining and biosynthesis of fumitremorgin-type alkaloids in ascomycetes. J. Antibiot. (Tokyo) 64, 45–49 (2011).
Maiya, S., Grundmann, A., Li, S.M. & Turner, G. The fumitremorgin gene cluster of Aspergillus fumigatus: identification of a gene encoding brevianamide F synthetase. ChemBioChem 7, 1062–1069 (2006).
Grundmann, A. & Li, S.M. Overproduction, purification and characterization of FtmPT1, a brevianamide F prenyltransferase from Aspergillus fumigatus. Microbiology 151, 2199–2207 (2005).
Kato, N. et al. Identification of cytochrome P450s required for fumitremorgin biosynthesis in Aspergillus fumigatus. ChemBioChem 10, 920–928 (2009).
Kato, N., Suzuki, H., Okumura, H., Takahashi, S. & Osada, H. A point mutation in ftmD blocks the fumitremorgin biosynthetic pathway in Aspergillus fumigatus strain Af293. Biosci. Biotechnol. Biochem. 77, 1061–1067 (2013).
Zhang, H., Wang, Y. & Pfeifer, B.A. Bacterial hosts for natural product production. Mol. Pharm. 5, 212–225 (2008).
Xu, W., Cai, X., Jung, M.E. & Tang, Y. Analysis of intact and dissected fungal polyketide synthase–nonribosomal peptide synthetase in vitro and in Saccharomyces cerevisiae. J. Am. Chem. Soc. 132, 13604–13607 (2010).
Hawkins, K.M. & Smolke, C.D. Production of benzylisoquinoline alkaloids in Saccharomyces cerevisiae. Nat. Chem. Biol. 4, 564–573 (2008).
Minami, H. et al. Microbial production of plant benzylisoquinoline alkaloids. Proc. Natl. Acad. Sci. USA 105, 7393–7398 (2008).
Ro, D.K. et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440, 940–943 (2006).
Lubertozzi, D. & Keasling, J.D. Developing Aspergillus as a host for heterologous expression. Biotechnol. Adv. 27, 53–75 (2009).
Ishiuchi, K. et al. Establishing a new methodology for genome mining and biosynthesis of polyketides and peptides through yeast molecular genetics. ChemBioChem 13, 846–854 (2012).
Meyer, V. et al. Highly efficient gene targeting in the Aspergillus niger kusA mutant. J. Biotechnol. 128, 770–775 (2007).
Cui, C.B., Kakeya, H., Okada, G., Onose, R. & Osada, H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus. I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. (Tokyo) 49, 527–533 (1996).
Takahashi, C. et al. Fumiquinazolines A–G, novel metabolites of a fungus separated from a Pseudolabrus marine fish. J. Chem. Soc. Perkin Trans. I 2345–2353 (1995).
Ames, B.D., Liu, X. & Walsh, C.T. Enzymatic processing of fumiquinazoline F: a tandem oxidative-acylation strategy for the generation of multicyclic scaffolds in fungal indole alkaloid biosynthesis. Biochemistry 49, 8564–8576 (2010).
Gao, X. et al. Fungal indole alkaloid biosynthesis: genetic and biochemical investigation of the tryptoquialanine pathway in Penicillium aethiopicum. J. Am. Chem. Soc. 133, 2729–2741 (2011).
Haynes, S.W., Ames, B.D., Gao, X., Tang, Y. & Walsh, C.T. Unraveling terminal C-domain–mediated condensation in fungal biosynthesis of imidazoindolone metabolites. Biochemistry 50, 5668–5679 (2011).
Li, S. et al. Biochemical characterization of NotB as an FAD-dependent oxidase in the biosynthesis of notoamide indole alkaloids. J. Am. Chem. Soc. 134, 788–791 (2012).
García-Estrada, C. et al. A single cluster of coregulated genes encodes the biosynthesis of the mycotoxins roquefortine C and meleagrin in Penicillium chrysogenum. Chem. Biol. 18, 1499–1512 (2011).
Grubbs, A.W., Artman, G.D. III, Tsukamoto, S. & Williams, R.M. A concise total synthesis of the notoamides C and D. Angew. Chem. Int. Edn Engl. 46, 2257–2261 (2007).
Kato, H. et al. Study on the biosynthesis of the notoamides: pinacol-type rearrangement of the isoprenyl unit in deoxybrevianamide E and 6-hydroxydeoxybrevianamide E. Tetrahedr. Lett. 52, 6923–6926 (2011).
Sanz-Cervera, J.F., Glinka, T. & Williams, R.M. Biosynthesis of brevianamides A and B: in search of the biosynthetic Diels-Alder construction. Tetrahedron 49, 8471–8482 (1993).
Wang, P., Gao, X. & Tang, Y. Complexity generation during natural product biosynthesis using redox enzymes. Curr. Opin. Chem. Biol. 16, 362–369 (2012).
Ishiuchi, K. et al. Combinatorial generation of complexity by redox enzymes in the chaetoglobosin A biosynthesis. J. Am. Chem. Soc. 135, 7371–7377 (2013).
Lazos, O. et al. Biosynthesis of the putative siderophore erythrochelin requires unprecedented crosstalk between separate nonribosomal peptide gene clusters. Chem. Biol. 17, 160–173 (2010).
Yin, W.B. et al. A nonribosomal peptide synthetase-derived iron(iii) complex from the pathogenic fungus Aspergillus fumigatus. J. Am. Chem. Soc. 135, 2064–2067 (2013).
Myers, E.W. & Miller, W. Optimal alignments in linear space. Comput. Appl. Biosci. 4, 11–17 (1988).
Ames, B.D. et al. Complexity generation in fungal peptidyl alkaloid biosynthesis: oxidation of fumiquinazoline A to the heptacyclic hemiaminal fumiquinazoline C by the flavoenzyme Af12070 from Aspergillus fumigatus. Biochemistry 50, 8756–8769 (2011).
Ohnishi, K. & Ono, B. Inverted repeat of a large segment unveiled on the right arm of Saccharomyces cerevisiae chromosome II. Yeast 22, 321–336 (2005).
Brachmann, C.B. et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14, 115–132 (1998).
Jones, E.W. Tackling the protease problem in Saccharomyces cerevisiae. Methods Enzymol. 194, 428–453 (1991).
Ma, S.M. et al. Complete reconstitution of a highly reducing iterative polyketide synthase. Science 326, 589–592 (2009).
Pompon, D., Louerat, B., Bronine, A. & Urban, P. Yeast expression of animal and plant P450s in optimized redox environments. Methods Enzymol. 272, 51–64 (1996).
Acknowledgements
We would like to thank J. Shimokawa at the University of Tokyo for providing us with a sample of spirotryprostatin A. We would like to express our appreciation to financial support from Japan Society for the Promotion of Science (JSPS) through the 'Funding Program for Next Generation World-Leading Researchers', initiated by the Council for Science and Technology Policy (no. LS103) (K.W.) and by the Industrial Technology Research Grant Program in 2009 (no. 09C46001a) from the New Energy and Industrial Technology Development Organization (NEDO) of Japan (K.W.). These works were also supported in part by The Uehara Memorial Foundation (K.W.), by Mochida Memorial Foundation for Medical and Pharmaceutical Research (K.W.), by The Hokuto Foundation for Bioscience (K.W.) and by The Naito Foundation Japan (K.W.). Postdoctoral fellowships to Y.T. from JSPS are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Y.T., N.I., H.M. and K.W. conceived and designed the study. Y.T. and N.I. designed and performed molecular cloning. Y.T. and N.I. performed the heterologous protein expression and purification as well as in vitro and in vivo characterization of the enzymes. D. W. and Y. G. elucidated the chemical structures. All of the authors analyzed and discussed the results. K.H. and K.W. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–68, Supplementary Tables 1–14 and Supplementary Notes 1–3. (PDF 7469 kb)
Rights and permissions
About this article
Cite this article
Tsunematsu, Y., Ishikawa, N., Wakana, D. et al. Distinct mechanisms for spiro-carbon formation reveal biosynthetic pathway crosstalk. Nat Chem Biol 9, 818–825 (2013). https://doi.org/10.1038/nchembio.1366
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1366
This article is cited by
-
Elucidation of genes enhancing natural product biosynthesis through co-evolution analysis
Nature Metabolism (2024)
-
Hydroxytryptophan biosynthesis by a family of heme-dependent enzymes in bacteria
Nature Chemical Biology (2023)
-
Unexpected assembly machinery for 4(3H)-quinazolinone scaffold synthesis
Nature Communications (2022)
-
Saccharomyces cerevisiae as host for the recombinant production of polyketides and nonribosomal peptides
Microbial Cell Factories (2021)
-
Structural basis of the stereoselective formation of the spirooxindole ring in the biosynthesis of citrinadins
Nature Communications (2021)