Abstract
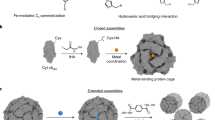
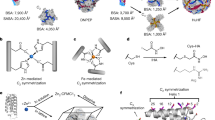
The ability to chemically control protein-protein interactions would allow the interrogation of dynamic cellular processes and lead to a better understanding and exploitation of self-assembling protein architectures. Here we introduce a new engineering strategy—reverse metal-templated interface redesign (rMeTIR)—that transforms a natural protein-protein interface into one that only engages in selective response to a metal ion. We have applied rMeTIR to render the self-assembly of the cage-like protein ferritin controllable by divalent copper binding, which has allowed the study of the structure and stability of the isolated ferritin monomer, the demonstration of the primary role of conserved hydrogen-bonding interactions in providing geometric specificity for cage assembly and the uniform chemical modification of the cage interior under physiological conditions. Notably, copper acts as a structural template for ferritin assembly in a manner that is highly reminiscent of RNA sequences that template virus capsid formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Klemm, J.D., Schreiber, S.L. & Crabtree, G.R. Dimerization as a regulatory mechanism in signal transduction. Annu. Rev. Immunol. 16, 569–592 (1998).
Fegan, A., White, B., Carlson, J.C.T. & Wagner, C.R. Chemically controlled protein assembly: techniques and applications. Chem. Rev. 110, 3315–3336 (2010).
Corson, T.W., Aberle, N. & Crews, C.M. Design and applications of bifunctional small molecules: Why two heads are better than one. ACS Chem. Biol. 3, 677–692 (2008).
Jones, S. & Thornton, J.M. Principles of protein-protein interactions. Proc. Natl. Acad. Sci. USA 93, 13–20 (1996).
Kortemme, T. & Baker, D. Computational design of protein-protein interactions. Curr. Opin. Chem. Biol. 8, 91–97 (2004).
Spencer, D.M., Wandless, T.J., Schreiber, S.L. & Crabtree, G.R. Controlling signal transduction with synthetic ligands. Science 262, 1019–1024 (1993).
Farrar, M.A., Alberola-lla, J. & Perlmutter, R.M. Activation of the Raf-1 kinase cascade by coumermycin-induced dimerization. Nature 383, 178–181 (1996).
Lin, H., Abida, W.M., Sauer, R.T. & Cornish, V.W. Dexamethasone-methotrexate: an efficient chemical inducer of protein dimerization in vivo. J. Am. Chem. Soc. 122, 4247–4248 (2000).
Gendreizig, S., Kindermann, M. & Johnsson, K. Induced protein dimerization in vivo through covalent labeling. J. Am. Chem. Soc. 125, 14970–14971 (2003).
Stankunas, K. et al. Conditional protein alleles using knockin mice and a chemical inducer of dimerization. Mol. Cell 12, 1615–1624 (2003).
Hussey, S.L., Muddana, S.S. & Peterson, B.R. Synthesis of a beta-estradiol-biotin chimera that potently heterodimerizes estrogen receptor and streptavidin proteins in a yeast three-hybrid system. J. Am. Chem. Soc. 125, 3692–3693 (2003).
Guo, Z., Zhou, D.M. & Schultz, P.G. Designing small-molecule switches for protein-protein interactions. Science 288, 2042–2045 (2000).
Salgado, E.N., Faraone-Mennella, J. & Tezcan, F.A. Controlling protein-protein interactions through metal coordination: assembly of a 16-helix bundle protein. J. Am. Chem. Soc. 129, 13374–13375 (2007).
Salgado, E.N., Lewis, R.A., Faraone-Mennella, J. & Tezcan, F.A. Metal-mediated self-assembly of protein superstructures: Influence of secondary interactions on protein oligomerization and aggregation. J. Am. Chem. Soc. 130, 6082–6084 (2008).
Salgado, E.N., Lewis, R.A., Mossin, S., Rheingold, A.L. & Tezcan, F.A. Control of protein oligomerization symmetry by metal coordination: C2 and C3 symmetrical assemblies through CuII and NiII coordination. Inorg. Chem. 48, 2726–2728 (2009).
Laganowsky, A. et al. An approach to crystallizing proteins by metal-mediated synthetic symmetrization. Protein Sci. 20, 1876–1890 (2011).
Liu, X. & Theil, E.C. Ferritins: Dynamic management of biological iron and oxygen chemistry. Acc. Chem. Res. 38, 167–175 (2005).
Tanaka, S. et al. Atomic-level models of the bacterial carboxysome shell. Science 319, 1083–1086 (2008).
Douglas, T. & Young, M. Viruses: making friends with old foes. Science 312, 873–875 (2006).
Zlotnick, A. To build a virus capsid—an equilibrium-model of the self-assembly of polyhedral protein complexes. J. Mol. Biol. 241, 59–67 (1994).
Stefanini, S., Vecchini, P. & Chiancone, E. On the mechanism of horse spleen apoferritin assembly–a sedimentation-velocity and circular-dichroism study. Biochemistry 26, 1831–1837 (1987).
Gerl, M., Jaenicke, R., Smith, J.M.A. & Harrison, P.M. Self-assembly of apoferritin from horse spleen after reversible chemical modification with 2,3-dimethylmaleic anhydride. Biochemistry 27, 4089–4096 (1988).
Vriezema, D.M. et al. Self-assembled nanoreactors. Chem. Rev. 105, 1445–1489 (2005).
Wong, K.K.W., Douglas, T., Gider, S., Awschalom, D.D. & Mann, S. Biomimetic synthesis and characterization of magnetic proteins (magnetoferritin). Chem. Mater. 10, 279–285 (1998).
Butts, C.A. et al. Directing noble metal ion chemistry within a designed ferritin protein. Biochemistry 47, 12729–12739 (2008).
Abe, S. et al. Polymerization of phenylacetylene by rhodium complexes within a discrete space of apo-ferritin. J. Am. Chem. Soc. 131, 6958–6960 (2009).
Aime, S., Frullano, L. & Crich, S.G. Compartmentalization of a gadolinium complex in the apoferritin cavity: a route to obtain high relaxivity contrast agents for magnetic resonance imaging. Angew. Chem. Int. Ed. Engl. 41, 1017–1019 (2002).
Uchida, M. et al. Targeting of cancer cells with ferrimagnetic ferritin cage nanoparticles. J. Am. Chem. Soc. 128, 16626–16633 (2006).
Comellas-Aragonès, M. et al. A virus-based single-enzyme nanoreactor. Nat. Nanotechnol. 2, 635–639 (2007).
Wörsdörfer, B., Woycechowsky, K.J. & Hilvert, D. Directed evolution of a protein container. Science 331, 589–592 (2011).
Uchida, M. et al. Biological containers: protein cages as multifunctional nanoplatforms. Adv. Mater. 19, 1025–1042 (2007).
Johnson, E., Cascio, D., Sawaya, M.R., Gingery, M. & Schroeder, I. Crystal structures of a tetrahedral open pore ferritin from the hyperthermophilic archaeon Archaeoglobus fulgidus. Structure 13, 637–648 (2005).
Bancroft, J.B. The self-assembly of spherical plant viruses. Adv. Virus Res. 16, 99–134 (1970).
Dalmau, M., Lim, S.R. & Wang, S.W. Design of a pH-dependent molecular switch in a caged protein platform. Nano Lett. 9, 160–166 (2009).
Lawson, D.M. et al. Solving the structure of human H-ferritin by genetically engineering intermolecular crystal contacts. Nature 349, 541–544 (1991).
Webb, B., Frame, J., Zhao, Z., Lee, M.L. & Watt, G.D. Molecular entrapment of small molecules within the interior of horse spleen ferritin. Arch. Biochem. Biophys. 309, 178–183 (1994).
Brodin, J.D. et al. Evolution of metal selectivity in templated protein interfaces. J. Am. Chem. Soc. 132, 8610–8617 (2010).
Salgado, E.N. et al. Metal-templated design of protein interfaces. Proc. Natl. Acad. Sci. USA 107, 1827–1832 (2010).
Hempstead, P.D. et al. Comparison of the three-dimensional structures of recombinant human H and horse L ferritins at high resolution. J. Mol. Biol. 268, 424–448 (1997).
Santambrogio, P. et al. Effects of modifications near the 2-, 3- and 4-fold symmetry axes an human ferritin renaturation. Biochem. J. 322, 461–468 (1997).
Zhang, Y. & Orner, B.P. Self-assembly in the ferritin nano-cage protein superfamily. Int. J. Mol. Sci. 12, 5406–5421 (2011).
Waldron, K.J. & Robinson, N.J. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 7, 25–35 (2009).
Swift, J. et al. Design of functional ferritin-like proteins with hydrophobic cavities. J. Am. Chem. Soc. 128, 6611–6619 (2006).
Mines, G.A., Pascher, T., Lee, S.C., Winkler, J.R. & Gray, H.B. Cytochrome c folding triggered by electron transfer. Chem. Biol. 3, 491–497 (1996).
Jones, T., Spencer, R. & Walsh, C. Mechanism and kinetics of iron release from ferritin by dihydroflavins and dihydroflavin analogs. Biochemistry 17, 4011–4017 (1978).
Treffry, A., Hawkins, C., Williams, J.M., Guest, J.R. & Harrison, P.M. Lability of iron at the dinuclear centres of ferritin studied by competition with four chelators. J. Biol. Inorg. Chem. 1, 49–60 (1996).
Yang, D. & Nagayama, K. Permeation of small molecules into the cavity of ferritin as revealed by proton nuclear-magnetic-resonance relaxation. Biochem. J. 307, 253–256 (1995).
Takagi, H., Shi, D.S., Ha, Y., Allewell, N.M. & Theil, E.C. Localized unfolding at the junction of three ferritin subunits - A mechanism for iron release? J. Biol. Chem. 273, 18685–18688 (1998).
Butler, P.J.G. Assembly of regular viruses. Int. Rev. Biochem. 25, 205–237 (1979).
Frausto da Silva, J.J.R. & Williams, R.J.P The Biological Chemistry of the Elements (Oxford University Press, Oxford, 2001).
Blackwell, J.R. & Horgan, R. A novel strategy for production of a highly expressed recombinant protein in an active form. FEBS Lett. 295, 10–12 (1991).
Schuck, P. A model for sedimentation in inhomogeneous media. I. Dynamic density gradients from sedimenting co-solutes. Biophys. Chem. 108, 187–200 (2004).
García de la Torre, J., Huertas, M.L. & Carrasco, B. Calculation of hydrodynamic properties of globular proteins from their atomic-level structure. Biophys. J. 78, 719–730 (2000).
Pace, N.C., Shirley, B.A. & Thomson, J.A. in Protein Structure: A Practical Approach (ed. Creighton, T.F.) 311–330 (IRL Press, Oxford, 1990).
John, D.M. & Weeks, K.M. van't Hoff enthalpies without baselines. Protein Sci. 9, 1416–1419 (2000).
Winn, M.D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Vagin, A. & Teplyakov, A. MOLREP: an automated program for molecular replacement. J. Appl. Crystallogr. 30, 1022–1025 (1997).
Murshudov, G.N., Vagin, A. & Dodson, E. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
DeLano, W.L. The PYMOL Molecular Graphics System (http://www.pymol.org/), 2003.
Laskowski, R.A., Macarthur, M.W., Moss, D.S. & Thornton, J.M. Procheck—A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993).
Acknowledgements
This work was supported by the US Department of Energy (Division of Materials Sciences, Office of Basic Energy Sciences, Award DE-FG02-10ER46677 to F.A.T.). F.A.T. was additionally supported by the Beckman Foundation, the Sloan Foundation and the National Science Foundation (CHE-0908115). Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource, operated by Stanford University on behalf of the Department of Energy. We thank J. Brodin for experimental assistance with TEM measurements and J. Faraone Mennella for illustrations.
Author information
Authors and Affiliations
Contributions
D.J.E.H. designed and performed experiments, analyzed data and co-wrote the paper. K.M.K. performed experiments. F.A.T. conceived and directed the project, analyzed data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results (PDF 8656 kb)
Rights and permissions
About this article
Cite this article
Huard, D., Kane, K. & Tezcan, F. Re-engineering protein interfaces yields copper-inducible ferritin cage assembly. Nat Chem Biol 9, 169–176 (2013). https://doi.org/10.1038/nchembio.1163
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1163
This article is cited by
-
Complete and cooperative in vitro assembly of computationally designed self-assembling protein nanomaterials
Nature Communications (2021)
-
Design and site-directed compartmentalization of gold nanoclusters within the intrasubunit interfaces of ferritin nanocage
Journal of Nanobiotechnology (2019)
-
Disulfide-mediated conversion of 8-mer bowl-like protein architecture into three different nanocages
Nature Communications (2019)
-
Engineering protein assemblies with allosteric control via monomer fold-switching
Nature Communications (2019)
-
Molecular cloning, expression and characterization of secreted ferritin in the silkworm, Bombyx mori
BioMetals (2019)