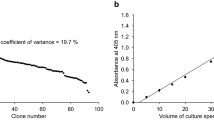
Abstract
Enterokinase (EK) is a heterodimeric serine protease which plays a key role in initiating the proteolytic digestion cascade in the mammalian duodenum. The enzyme acts by converting trypsinogen to trypsin via a highly specific cleavage following the pentapeptide recognition sequence (Asp)4-Lys. This stringent site specificity gives EK great potential as a fusion protein cleavage reagent. Recently, a cDNA encoding the catalytic (light) chain of bovine enterokinase (EKL) was identified, characterized, and transiently expressed in mammalian COS cells. We report here the production of EKL in Escherichia coli by a novel secretory expression system that utilizes E. coli DsbA protein as an N-terminal fusion partner. The EKL cDNA was fused in-frame to the 3′-end of the coding sequence for DsbA, with the two domains of the fusion protein separated by a linker sequence encoding an enterokinase recognition site. Active, processed recombinant EKL, (rEKL) was generated from this fusion protein via an autocatalytic cleavage reaction. The enzymatic properties of the bacterially produced rEKL were indistinguishable from the previously described COS-derived enzyme. Both forms of rEKL were capable of cleaving peptides, polypeptides and trypsinogen with the same specificity exhibited by the native heterodimeric enzyme purified from bovine duodena. Interestingly, rEKL activated trypsinogen poorly relative to the native heterodimeric enzyme, but was superior in its ability to cleave artificial fusion proteins containing the (Asp)4-Lys recognition sequence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Maina, C.V., Riggs, P.D., Grandea, A.G., Slatko, E.E., Moran, L.S., Tagliamonte, J.A., McReynolds, L.A. and Guan, C. 1988. An Escherichia coli vector to express and purify foreign proteins by fusion to and separation from maltose-binding protein. Gene 74: 365–373.
Smith, D.B. and Johnson, K.S. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione-S-transferase. Gene 67: 3l–40.
LaVallie, E.R., DiBlasio, E.A., Kovacic, S., Grant, K.L., Schendel, P.F. and McCoy, J.M. 1993. A thioredoxin gene fusion system that circumvents inclusion body formation in the E. coli cytoplasm. Bio/Technology 11: 187–193.
Nagai, K. and Thøgersen, H.C. 1984. Generation of β-globin by sequence-specific proteolysis of a hybrid protein in Escherichia coli. Nature 309: 810–812.
Chang, J.-Y. 1985. Thrombin specificity. Requirement for apolar amino acids adjacent to the thrombin cleavage site of polypeptide substrate. Eur. J. Biochem. 151: 217–224.
Maroux, S., Baratti, J. and Desnuelle, R. 1971. Purification and specificity of porcine enterokinase. J. Biol. Chem. 246: 5031–5039.
Liepnieks, J.J. and Light, A. 1979. The preparation and properties of bovine enterokinase. J. Biol. Chem. 254: 1677–1683.
LaVallie, E.R., Rehemtulla, A., Racie, L.A., DiBlasio, E.A., Ferenz, C., Grant, K.L., Light, A. and McCoy, J.M. 1993. Cloning and functional expression of a cDNA encoding the catalytic subunit of bovine enterokinase. J. Biol. Chem. 268: 23311–23317.
Kunitz, M. 1939. Formation of trypsin from crystalline trypsinogen by means of enterokinase. J. Gen. Physiol. 22: 429–446.
Bricteux-Gregoire, S., Schyns, R. and Florkin, M. 1972. Phylogeny of trypsinogen activation peptides. Comp. Biochem. Physiol. 42B: 23–39.
Hopp, T.P., Prickett, K.S., Price, V.L., Libby, R.T., March, C.J., Cerretti, D.P., Urdal, D.L. and Conlon, P.J. 1988. A short polypeptide marker sequence useful for recombinant protein identification and purification. Bio/Technology. 6: 1204–1210.
Bardwell, J.C., McGovern, K. and Beckwith, J. 1991. Identification of a protein required for disulfide bond formation in vivo. Cell 67: 581–589.
Martin, J.L., Bardwell, J.C.A. and Kuriyan, J. 1993. Crystal structure of the DsbA protein required for disulfide bond formation in vivo. Nature 365: 464–468.
Janknecht, R., de Martynoff, G., Lou, J., Hipskind, R. A., Nordheim, A. and Stunnenberg, H.G. 1991. Rapid and efficient purification of native histidine-tagged protein expressed by recombinant vaccinia virus. Proc. Natl. Acad. Sci. USA 88: 8972–8976.
Light, A. and Fonseca, P. 1984. The preparation and properties of the catalytic subunit of bovine enterokinase. J. Biol. Chem. 259: 13195–13198.
Dykes, C.W., Bookless, A.B., Coomber, B.A., Noble, S.A., Humber, D.C. and Hobden, A.N. 1988. Expression of atrial natriuretic factor as a cleavable fusion protein with chloramphenicol acetyltransferase in Escherichia coli. Eur. J. Biochem 174: 411–416.
Forsberg, G., Baastrup, B., Rondahl, H., Holmgren, E., Pohl, G., Hartmanis, M. and Lake, M. 1992. An evaluation of different enzymatic cleavage methods for recombinant fusion proteins, applied on des (1-3) insulin-like growth factor I. J. Prot. Chem. 11: 201–211.
Hirel, P-H., Schmitter, J.-M., Dessen, P., Fayat, G. and Blanquet, S. 1989. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc. Natl. Acad. Sci. USA 86: 8247–8251.
Fehlhammer, H., Bode, W. and Huber, R. 1977. Crystal structure of bovine trypsinogen at 1.8 Å resolution. J. Mol. Biol. 11: 415–438.
Derman, A.I., Prinz, W.A., Belin, D. and Beckwith, J. 1993. Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli Science 262: 1744–1747.
Vasquez, J.R., Evnin, L.B., Higaki, J.N. and Craik, C.S. 1989. An expression system for trypsin. J. Cell. Biochem. 39: 265–276.
Corey, D.R. and Craik, C.S. 1992. An investigation into the minimum requirements for peptide hydrolysis by mutation of the catalytic triad of trypsin. J. Am. Chem. Soc. 114: 1784–1790.
Rehemtulla, A., Dorner, A.J. and Kaufman, R.J. 1992. Regulation of PACE propeptide-processing activity: requirement for a post-endoplasmic reticulum compartment and autoproteolytic activation. Proc. Natl. Acad. Sci. USA 89: 8235–8239.
Light, A., Savithri, H.S. and Liepnieks, J.J. 1980. Specificity of bovine enterokinase toward protein substrates. Anal. Biochem. 106: 199–206.
Guan, C., Li, P., Riggs, P.D. and Inouye, H. 1988. Vectors that facilitate the expression and purification of foreign peptides in Escherichia coli by fusion to maltose-binding protein. Gene 67: 21–30.
Sambrook, J., Fritsch, E.F. and Maniatis, J. 1989. Molecular Cloning, a Laboratory Manual, second edition, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
Dower, W.J., Miller, J.F. and Ragsdale, C.W. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16: 6127–6145.
Grant, D.A.W. and Hermon-Taylor, J. 1979. Hydrolysis of artificial substrates by enterokinase and trypsin and the development of a sensitive specific assay for enterokinase in serum. Biochim. Biophys. Acta. 567: 207–215.
Mullis, K., Faloona, F., Scharf, S., Saiki, R., Horn, G. and Erlich, H. 1986. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symp. Quant. Biol. 51: 263–273.
Saiki, R.K., Gelfand, D.H., Stoffel, S., Scharf, S.J., Higuchi, R., Horn, G.T., Mullis, K.B. and Erlich, H.A. 1988. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239: 487–494.
Wilson, K. 1990. Preparation of genomic DNA from bacteria. Current Protocols in Molecular Biology (suppl. 9): 2.4.1–2.4.2.
Gill, S.C. and von Hippel, P.H. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182: 319–326.
Duplay, P., Bedouelle, H., Fowler, A., Zabin, I., Saurin, W. and Hofnung, M. 1984. Sequences of the malE gene and its product, the maltose-binding protein of Escherichia coli K12. J. Biol. Chem. 259: 10606–10613.
Schagger, H. and von Jagow, G. 1987. Tricine-sodium dodecyl sulfate-poly-acrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100kDa. Anal. Biochem. 166: 368–379.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Collins-Racie, L., McColgan, J., Grant, K. et al. Production of Recombinant Bovine Enterokinase Catalytic Subunit in Escherichia coli Using the Novel Secretory Fusion Partner DsbA. Nat Biotechnol 13, 982–987 (1995). https://doi.org/10.1038/nbt0995-982
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0995-982
This article is cited by
-
A simple and efficient method for cytoplasmic production of human enterokinase light chain in E. coli
AMB Express (2022)
-
Comparison of Periplasmic and Cytoplasmic Expression of Bovine Enterokinase Light Chain in E. coli
The Protein Journal (2022)
-
Production of autolysis-proof Kex2 protease from Candida albicans in Saccharomyces cerevisiae for in vitro processing of fusion proteins
Applied Microbiology and Biotechnology (2022)
-
Thermococcus sp. KS-1 PPIase as a fusion partner improving soluble production of aromatic amino acid decarboxylase
AMB Express (2021)
-
Protein fusion tags for efficient expression and purification of recombinant proteins in the periplasmic space of E. coli
3 Biotech (2016)