Abstract
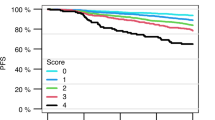
The purpose was to assess predictive factors for outcome in patients with chronic myeloid leukemia (CML) in chronic phase (CML-CP) treated with nilotinib after imatinib failure. Imatinib-resistant and -intolerant patients with CML-CP (n=321) were treated with nilotinib 400 mg twice daily. Of 19 baseline patient and disease characteristics and two response end points analyzed, 10 independent prognostic factors were associated with progression-free survival (PFS). In the multivariate analysis, major cytogenetic response (MCyR) within 12 months, baseline hemoglobin ⩾120 g/l, baseline basophils <4%, and absence of baseline mutations with low sensitivity to nilotinib were associated with PFS. A prognostic score was created to stratify patients into five groups (best group: 0 of 3 unfavorable risk factors and MCyR by 12 months; worst group: 3 of 3 unfavorable risk factors and no MCyR by 12 months). Estimated 24-month PFS rates were 90%, 79%, 67% and 37% for patients with prognostic scores of 0, 1, 2 and 3, respectively, (no patients with score of 4). Even in the presence of poor disease characteristics, nilotinib provided significant clinical benefit in patients with imatinib-resistant or -intolerant CML. This system may yield insight on the prognosis of patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jabbour E, Cortes JE, Giles FJ, O’Brien S, Kantarjian HM . Current and emerging treatment options in chronic myeloid leukemia. Cancer 2007; 109: 2171–2181.
Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med 2002; 346: 645–652.
O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348: 994–1004.
Cortes J, Talpaz M, O’Brien S, Jones D, Luthra R, Shan J et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res 2005; 11: 3425–3432.
Kantarjian HM, Talpaz M, O'Brien S, Jones D, Giles F, Garcia-Manero G et al. Survival benefit with imatinib mesylate versus interferon-alpha-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood 2006; 108: 1835–1840.
Deininger M, O’Brien SG, Guilhot F, Goldman JM, Hochhaus A, Hughes TP et al. International Randomized Study of Interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. ASH Ann Meeting Abstracts 2009; 114: 1126.
Shah NP . Loss of response to imatinib: mechanisms and management. Hematol Am Soc Hematol Educ Program 2005, 183–187.
Hochhaus A, Hughes T . Clinical resistance to imatinib: mechanisms and implications. Hematol Oncol Clin North Am 2004; 18: 641–656,, ix.
Tasigna (nilotinib) prescribing information Novartis Pharmaceuticals Corporation: East Hanover, NJ, 2012.
Kantarjian HM, Giles F, Gattermann N, Bhalla K, Alimena G, Palandri F et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood 2007; 110: 3540–3546.
Kantarjian HM, Giles FJ, Bhalla KN, Pinilla-Ibarz JA, Larson RA, Gattermann N et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase following imatinib resistance or intolerance: 24-month follow-up results. Blood 2011; 117: 1141–1145.
Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009; 27: 6041–6051.
Kantarjian HM, Talpaz M, O'Brien S, Smith TL, Giles FJ, Faderl S et al. Imatinib mesylate for Philadelphia chromosome-positive, chronic-phase myeloid leukemia after failure of interferon-alpha: follow-up results. Clin Cancer Res 2002; 8: 2177–2187.
Tam CS, Kantarjian H, Garcia-Manero G, Borthakur G, O'Brien S, Ravandi F et al. Failure to achieve a major cytogenetic response by 12 months defines inadequate response in patients receiving nilotinib or dasatinib as second or subsequent line therapy for chronic myeloid leukemia. Blood 2008; 112: 516–518.
Milojkovic D, Nicholson E, Apperley JF, Holyoake TL, Shepherd P, Drummond MW et al. Early prediction of success or failure of treatment with second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukemia. Haematologica 2010; 95: 224–231.
Anderson JR, Cain KC, Gelber RD . Analysis of survival by tumor response. J Clin Oncol 1983; 1: 710–719.
Anderson JR, Cain KC, Gelber RD . Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol 2008; 26: 3913–3915.
Buyse M, Piedbois P . On the relationship between response to treatment and survival time. Stat Med 1996; 15: 2797–2812.
Jabbour E, Kantarjian H, O’Brien S, Shan J, Garcia-Manero G, Wierda W et al. Predictive factors for outcome and response in patients treated with second-generation tyrosine kinase inhibitors for chronic myeloid leukemia in chronic phase after imatinib failure. Blood 2011; 117: 1822–1827.
Jabbour E, Jones D, Kantarjian HM, O’Brien S, Tam C, Koller C et al. Long-term outcome of patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors after imatinib failure is predicted by the in vitro sensitivity of BCR-ABL kinase domain mutations. Blood 2009; 114: 2037–2043.
Jabbour E, Bahceci E, Zhu C, Lambert A, Cortes J . Predictors of long-term cytogenetic response following dasatinib therapy of patients with chronic-phase chronic myeloid leukemia (CML-CP). ASH Ann Meeting Abstracts 2009; 114: 3296.
Acknowledgements
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Michael Mandola, PhD for medical editorial assistance with this manuscript.
Authors Contributions
EJ, JEC, FJG, JP-I, OGO, AH, TPH, JPR, D-WK, GM and HMK designed the study; HMK provided administrative support; EJ, PDlC, JC, FJG, JP-I, RAL, NG, OGO, TPH, JPR, D-WK, GM, MB and HMK provided study materials; EJ, PDlC, KNB, NG and AH collected and assembled data; EJ, JEC, AH, GS, D-WK, JR, RCW, MB and HMK analyzed and interpreted data; and all authors drafted/approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
EJ received honoraria from Novartis and BMS. PDlC acted as a consultant and received honoraria for Novartis and BMS and received research funding from Novartis. JEC acted as a consultant for Novartis, BMS and Pfizer and received research funding from Novartis, BMS, Pfizer, Ariad and Chemgenex. FJG acted as a consultant, received honoraria and research funding from Novartis. KNB received honoraria and research funding from Novartis. JP-I acted as a consultant for Novartis and BMS and received honoraria from Novartis. RAL acted as a consultant, received honoraria, and received research funding from Novartis. NG received honoraria and research funding from Novartis. OGO acted as a consultant, received honoraria, and research funding from Novartis. AH acted as a consultant for Novartis, BMS, Pfizer and Ariad, and received honoraria and research funding from Novartis, BMS and Pfizer. TPH acted as a consultant and received research funding from Novartis, BMS and Ariad. GS acted as a consultant for Novartis, BMS and Pfizer and received honoraria from BMS and Novartis. JPR acted as a consultant for Novartis, BMS, Ariad and Pfizer and received research funding from Novartis. D-WK received honoraria from Novartis and BMS and received research funding from Novartis, BMS, Pfizer and Ariad. GM acted as a consultant for Novartis, BMS, Pfizer and Genzyme, and received honoraria from Novartis and BMS; and research funding from Novartis. JR and RCW are Novartis employees and stock owners. MB acted as a consultant for Novartis, BMS and Pfizer, and received honoraria from Novartis, BMS and Pfizer, and received research funding from Novartis. HMK acted as a consultant for Novartis and received research funding from Novartis, BMS and Pfizer.
Additional information
Presented in abstract form at the 51st annual meeting of the American Society of Hematology, New Orleans, LA, December 7, 2009.
Rights and permissions
About this article
Cite this article
Jabbour, E., le Coutre, P., Cortes, J. et al. Prediction of outcomes in patients with Ph+ chronic myeloid leukemia in chronic phase treated with nilotinib after imatinib resistance/intolerance. Leukemia 27, 907–913 (2013). https://doi.org/10.1038/leu.2012.305
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.305
Keywords
This article is cited by
-
Prediction of response and survival in patients with chronic-phase chronic myeloid leukemia treated with omacetaxine mepesuccinate: logistic regression and landmark analyses
Blood Cancer Journal (2015)
-
Causes of resistance and treatment choices of second- and third-line treatment in chronic myelogenous leukemia patients
Annals of Hematology (2015)
-
MDR1 expression predicts outcome of Ph+ chronic phase CML patients on second-line nilotinib therapy after imatinib failure
Leukemia (2014)