Abstract
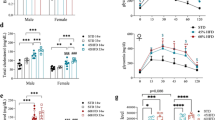
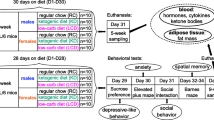
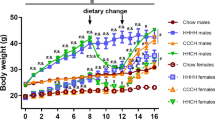
In this review, we discuss the observations that, following chronic high-fat diet (HFD) exposure, male mice have higher levels of saturated fatty acids (FAs) and total sphingolipids, whereas lower amounts of polyunsaturated FAs in the central nervous system (CNS) than females. Furthermore, males, when compared with female mice, have higher levels of inflammatory markers in the hypothalamus following exposure to HFD. The increase in markers of inflammation in male mice is possibly due to the reductions in proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) and estrogen receptor alpha (ERα), which is not recapitulated in female mice. Consistently, hypothalamic inflammation is induced both in male and female ERα total-body knockout mice when exposed to a HFD, thus confirming the key role of ERα in the regulation of HFD-induced hypothalamic inflammation. Finally, the HFD-induced depletion of hypothalamic ERα is associated with dysregulation in metabolic homeostasis, as evidenced by reductions in glucose tolerance and decrements in myocardial function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Shi H, Seeley RJ, Clegg DJ . Sexual differences in the control of energy homeostasis. Front Neuroendocrinol 2009; 30: 396–404.
Sugiyama MG, Agellon LB . Sex differences in lipid metabolism and metabolic disease risk. BiochemCell Biol 2012; 90: 124–141.
Ford ES . Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care 2005; 28: 2745–2749.
Kopelman PG . Obesity as a medical problem. Nature 2000; 404: 635–643.
Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS . Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA 2000; 97: 12729–12734.
Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 1994; 331: 1056–1061.
Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 2011; 14: 453–465.
Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA 2007; 104: 2501–2506.
Zhang L, Bruce-Keller AJ, Dasuri K, Nguyen AT, Liu Y, Keller JN . Diet-induced metabolic disturbances as modulators of brain homeostasis. Biochim Biophys Acta 2009; 1792: 417–422.
Hotamisligil GS . Inflammation and metabolic disorders. Nature 2006; 444: 860–867.
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2011; 122: 153–162.
Zhang QG, Wang R, Tang H, Dong Y, Chan A, Sareddy GR et al. Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus. Mol Cell Endocrinol 2014; 389: 84–91.
De Marinis E, Acaz-Fonseca E, Arevalo MA, Ascenzi P, Fiocchetti M, Marino M et al. 17beta-Oestradiol anti-inflammatory effects in primary astrocytes require oestrogen receptor beta-mediated neuroglobin up-regulation. J Neuroendocrinol 2013; 25: 260–270.
Petrone AB, Simpkins JW, Barr TL . 17beta-estradiol and inflammation: implications for ischemic stroke. Aging Dis 2014; 5: 340–345.
Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C et al. Hypothalamic PGC-1alpha protects against high-fat diet exposure by regulating ERalpha. Cell Rep 2014; 9: 633–645.
Summers SA . Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 2006; 45: 42–72.
Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab 2013; 17: 790–797.
Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009; 58: 337–343.
Borg ML, Omran SF, Weir J, Meikle PJ, Watt MJ . Consumption of a high-fat diet, but not regular endurance exercise training, regulates hypothalamic lipid accumulation in mice. J Physiol 2012; 590: 4377–4389.
Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 2011; 121: 1858–1870.
Langeveld M, Aerts JM . Glycosphingolipids and insulin resistance. Prog Lipid Res 2009; 48: 196–205.
Samad F, Hester KD, Yang G, Hannun YA, Bielawski J . Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes 2006; 55: 2579–2587.
Davis KE, D Neinast M, Sun K, M Skiles W, D Bills J, A Zehr J et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab 2013; 2: 227–242.
Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 2009; 296: E1003–E1012.
Opie LH, Walfish PG . Plasma free fatty acid concentrations in obesity. N Engl J Med 1963; 268: 757–760.
Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C et al. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA 2003; 100: 9614–9619.
Spence RD, Wisdom AJ, Cao Y, Hill HM, Mongerson CR, Stapornkul B et al. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERalpha signaling on astrocytes but not through ERbeta signaling on astrocytes or neurons. J Neurosci 2013; 33: 10924–10933.
Bourdoncle A, Labesse G, Margueron R, Castet A, Cavailles V, Royer CA . The nuclear receptor coactivator PGC-1alpha exhibits modes of interaction with the estrogen receptor distinct from those of SRC-1. J Mol Biol 2005; 347: 921–934.
Tcherepanova I, Puigserver P, Norris JD, Spiegelman BM, McDonnell DP . Modulation of estrogen receptor-alpha transcriptional activity by the coactivator PGC-1. J Biol Chem 2000; 275: 16302–16308.
Bazinet RP, Laye S . Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci 2014; 15: 771–785.
Luchtman DW, Song C . Cognitive enhancement by omega-3 fatty acids from child-hood to old age: findings from animal and clinical studies. Neuropharmacology 2013; 64: 550–565.
Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH . Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids 2000; 35: 1305–1312.
Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 2003; 60: 940–946.
Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol 2006; 63: 1545–1550.
Kiecolt-Glaser JK, Belury MA, Porter K, Beversdorf DQ, Lemeshow S, Glaser R . Depressive symptoms, omega-6:omega-3 fatty acids, and inflammation in older adults. Psychosom Med 2007; 69: 217–224.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Morselli, E., Frank, A., Palmer, B. et al. A sexually dimorphic hypothalamic response to chronic high-fat diet consumption. Int J Obes 40, 206–209 (2016). https://doi.org/10.1038/ijo.2015.114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.114
This article is cited by
-
Metabolic factors in the regulation of hypothalamic innate immune responses in obesity
Experimental & Molecular Medicine (2022)
-
Hypothalamic inflammation in metabolic disorders and aging
Cellular and Molecular Life Sciences (2022)
-
The hypothalamus for whole-body physiology: from metabolism to aging
Protein & Cell (2022)
-
Palmitic acid control of ciliogenesis modulates insulin signaling in hypothalamic neurons through an autophagy-dependent mechanism
Cell Death & Disease (2022)
-
Hypothalamic endocannabinoids in obesity: an old story with new challenges
Cellular and Molecular Life Sciences (2021)