Abstract
More than half of children with epilepsy outgrow their seizures, yet the underlying mechanism is unknown. GABAergic inhibition increases at puberty in female mice due to expression of extrasynaptic α4βδ GABAA receptors (GABARs). Therefore, we tested the role of these receptors in regulating seizure-like discharges in CA1 hippocampus using a high K+ (8.5 mM) seizure model. Spontaneous field potentials were recorded from hippocampus of pre-pubertal (~28–32 PND) and pubertal (~35–44 PND) female wild-type or α4−/− mice. The coastline length, a measure of burst intensity, was assessed. 8.5 mM K+ induced seizure-like discharges in over 60% of pre-pubertal slices, but only in 7% of pubertal slices, where the coastline length was reduced by 70% (P = 0.04). However, the pubertal decrease in seizure-like discharges was not seen in the α4−/−, implicating α4βδ GABARs as the cause of the decreased seizure-like activity during puberty. Administration of THIP or DS2, to selectively increase α4βδ current, reduced activity in 8.5 mM K+ at puberty, while blockade of α5-GABARs had no effect. GABAergic current was depolarizing but inhibitory in 8.5 mM K+, suggesting a mechanism for the effects of α4βδ and α5-GABARs, which exhibit different polarity-dependent desensitization. These data suggest that α4βδ GABARs are anti-convulsant during adolescence.
Similar content being viewed by others
Introduction
Epilepsy is a common neurological disorder in childhood. Epilepsy is more than twice as common in children as in adults (about 700 per 100,000 in children under the age of 16 years compared to 330 per 100,000 in adults)1. Furthermore, the incidence of status epilepticus in developed countries is between 17 and 23/100,000, with a higher incidence in younger children1. These skewed statistics reflect the fact that epilepsy frequently remits in adolescence2, an outcome which is more commonly noted for certain epilepsy syndromes3. However, the underlying mechanism for this marked decrease in seizure susceptibility during adolescence is not known.
One factor that has not yet been examined is the role of extrasynaptic GABAergic inhibition, which increases at the onset of puberty4. GABAA receptors (GABARs) are pentameric membrane proteins of diverse subtype which gate a Cl− conductance and are the most prevalent source of inhibition in the brain5. They are localized not only sub-synaptically, but certain subtypes, such as α4βδ, localize at extrasynaptic sites6 where they generate a tonic inhibition from ambient GABA due to their high affinity for GABA and relative lack of desensitization7 under steady-state conditions. Highest levels of expression of α4βδ GABARs are found in dentate gyrus granule cells8, where they generate a tonic inhibition9. However, the CA1 hippocampus, which normally exhibits very low levels of expression of this receptor exhibits a dramatic increase in expression during the pubertal period4,10. Previous studies from this laboratory have shown that expression of α4βδ GABARs increases 4 to 8-fold on the dendrites of CA1 hippocampal pyramidal cells at the onset of puberty in female mice from nearly undetectable levels assessed pre-pubertally4,11. This increase in tonic inhibition reduces neuronal excitability at puberty4. One would expect this increase in GABAergic inhibition during puberty to have an inhibitory effect on CA1 hippocampal network activity such as the production of epileptiform bursts. Therefore, we tested whether pubertal CA1 hippocampus would generate less seizure-like activity than pre-pubertal hippocampus and we examined the influence of various GABAR subtypes on spontaneous discharge in high K+ during puberty to test the hypothesis that α4βδ GABARs selectively reduce seizure-like activity at puberty.
To test neuronal excitability in the hippocampus, we exposed hippocampal slices to high K+ to elicit synchronized bursting in the CA1 region. This is a well-established in vitro model commonly used to elicit seizure-like activity12,13,14. The magnitude of the resultant epileptiform activity was assessed using the coastline length measurement, a measure of individual burst intensity (the summation of burst amplitude, duration and intra-burst frequency)12, across pubertal stages in wild-type and α4−/− mice.
Results
Pubertal slices are resistant to expression of seizure-like discharges
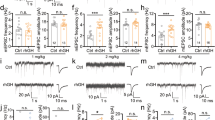
We perfused slices with high K+ (8.5 mM) to elicit seizure-like discharges12 and compared the response in pubertal vs. pre-pubertal hippocampus. All slices continuously bathed in 8.5 mM K+ developed a pattern of spontaneous synchronized bursting, the magnitude of which increased gradually, stabilized by 35–50 min and could be maintained for hours. Two types of discharges were observed: seizure-like (“ictal activity”) or synchronized discharge (“inter-ictal activity”)15. Seizure-like activity was observed in over 60% of the pre-pubertal hippocampal slices (9 out of 14 slices) but in only 7% of the pubertal slices (1 out of 14 slices, P = 0.005, Fig. 1) in 8.5 mM K+. The remainder of the pre-pubertal slices exhibited discharge patterns similar to the typical pubertal pattern. In addition, the coastline length was reduced by 70% at puberty compared to pre-pubertal values (pre-pub, 16.8 ± 5.2, pub, 5.4 ± 0.78, P = 0.04, Fig. 1), suggesting that increases in synaptic activity produced by the high K+ aCSF are reduced at puberty.
A high K+ seizure model triggers less seizure-like activity at puberty: Reversal by α4 knock-out.
(a) Representative 20 min traces illustrating the predominant response to 8.5 mM K+ for each group. Left, pre-pubertal WT, middle, pubertal WT, right, pubertal α4−/−. (b) Expansion of the time-base for indicated regions (blue arrow). (c) Averaged coastline length, a measure of neuronal activity, reveals greater activity pre-pubertally and in the pubertal α4−/−. *ANOVA, F(2, 32) = 3.50, P = 0.042 vs. other groups (100 s after 8.5 mM K+). (d) Percent of slices exhibiting seizure-like activity. *P = 0.005 vs. other groups, χ2 = 10.6, DF = 2. (n = 7 mice, α4−/−; n = 14 mice, WT Pre-pub; n = 14 mice, WT Pub).
Reduced expression of seizure-like discharges at puberty is not seen in the α4 knock-out
Because α4βδ GABARs are increased at puberty4,10, we tested whether knock-out of α4 would increase seizure-like activity at this time. To this end, we evaluated the coastline length for pubertal α4−/− hippocampal activity in 8.5 mM K+. This parameter was increased more than 100% above pubertal wild-type (WT) values (19.3 ± 4.4, P = 0.04, Fig. 1) and was not significantly different from pre-pubertal values. Seizure-like activity was observed in nearly 60% of the slices (4 out of 7 slices), similar to pre-pubertal assessments and significantly more likely to occur than in pubertal wild-type slices (P = 0.005, Fig. 1). These data suggest that the increased expression of α4βδ GABARs at puberty exerts an anti-convulsant effect.
Increasing α4βδ GABAR-gated current with THIP further reduces spontaneous discharge in high K+ at puberty
Because α4 knock-out increased seizure-like activity and the coastline length in pubertal hippocampus to pre-pubertal levels, we tested the effects of the GABA agonist THIP at a concentration selective for δ-containing GABARs7,16. We predicted that THIP would have a greater effect to reduce synaptic activity in pubertal hippocampus compared to pre-pubertal. Synchronized activity was recorded from CA1 hippocampus (pre-pubertal and pubertal) in 8.5 mM K+ before and after bath application of 1 μM THIP. THIP produced significant decreases in both the frequency (P = 0.0023) and amplitude (P = 2.5 × 10−5) of spontaneous events (Table 1), resulting in a 24% decrease in the coastline length (pre-THIP, 8.7 ± 1.2; post-THIP, 6.6 ± 1; Fig. 2, P = 2.07 × 10−6). In contrast, THIP had no significant effect on the coastline length in pre-pubertal hippocampus (pre-THIP, 15.2 ± 3.36; post-THIP, 14.5 ± 3.7; P = 0.24, Fig. 2). These results are consistent with the finding that the α4βδ GABARs exert anti-convulsant effects selectively at puberty, when expression of this receptor subtype is increased.
THIP selectively reduces spontaneous discharge in high K+ at puberty.
(a,b) Representative traces of spontaneous synchronized discharges recorded in 8.5 mM K+ from pre-pubertal (a) and pubertal (b) CA1 hippocampus before and after 1 μM THIP, which is selective for δ-GABARs. (c) Expanded time-base of traces (Pub) presented in (b). (*individual event from indicated trace) (d), Averaged values of the coastline length, #slices/group, n = 19 slices from 10 mice, Pre-pub; n = 15 slices from 10 mice, Pub. *(1-tail) paired t-test, t(14) = 7.26, P = 2.07 × 10−6 vs. pre-THIP. (e) Averaged ratios of the coastline length (CLL) for THIP relative to the pre-drug control. (2-tail) t-test, t(32) = 3.46, *P = 0.0016 vs. pre-pub.
Increasing α4βδ GABAR-gated current with DS2 further reduces spontaneous discharge in high K+ at puberty
We compared effects of another δ–selective GABA drug17, DS2 (10 μM), on epileptiform activity in high K+ aCSF at puberty. We also predicted that DS2 would have a greater effect to reduce synaptic activity in pubertal hippocampus compared to pre-pubertal. In fact, DS2 produced a significant 17% decrease in the coastline length (pre-DS2, 7.62 ± 0.7; post-DS2, 6.3 ± 0.7; Fig. 3, P = 2.2 × 10−6) at puberty, due to significant decreases in both the frequency (P = 0.00024) and amplitude (P = 1.29 × 10−6) of spontaneous events (Table 1). In contrast, bath application of this drug had no significant effect on the coastline length in pre-pubertal hippocampus (pre-DS2, 13.6 ± 3.4; post-DS2, 12.6 ± 2.9; P = 0.10, Fig. 3). These results are consistent with the finding that the α4βδ GABARs exert anti-convulsant effects selectively at puberty.
DS2 selectively reduces spontaneous discharge in high K+ at puberty.
(a,b) Representative traces of neuronal activity recorded in 8.5 mM K+ from pre-pubertal (a) and pubertal (b) CA1 hippocampus before and after 10 μM DS2, a GABA modulator which is selective for δ-GABARs. (c) Expanded time-base of traces (Pub) presented in (b). (*individual event from indicated trace) (d), Averaged values of the coastline length, *(1-tail) paired t-test, t(16) = 6.8, P = 2.15 × 10−6 vs. pre-DS2. (e) Averaged ratios of the coastline length (CLL) for DS2 relative to the pre-drug control. *(2-tail) t-test, t(33) = 3.85, P = 5.1 × 10−4 vs. pre-pub. n = 18 slices from 9 mice, Pre-pub; n = 18 slices from 9 mice, Pub.
Blockade of α5-GABARs does not increase spontaneous discharge in high K+at puberty
α5βγ2 GABARS are the predominant extrasynaptic GABAR subtype in the CA1 hippocampus where they generate a tonic inhibitory current18. Therefore, we tested whether blockade of this receptor with the selective inverse agonist, L-655,70818, would alter synaptic activity at puberty in 8.5 mM K+. To this end, neuronal activity was recorded in pubertal slices before and after bath application of 50 nM L-655,708 (IC50 = 20 nM)16. L-655,708 produced no significant change in the coastline length (pre-L-655,708, 7.3 ± 0.8; post-L655,708, 7.2 ± 1; P = 0.75, Fig. 4) or in the frequency or amplitude of spontaneous events (Table 1), suggesting that α5βγ2 GABARs do not contribute to the reduced seizure-like activity observed at puberty.
Blockade of α5-GABARs has no effect on spontaneous discharge in high K+ at puberty.
(a) Representative traces of spontaneous synchronized activity recorded in 8.5 mM K+ from pubertal CA1 hippocampus before (left) and after (right) 50 nM L-655,708 (L-655) to block α5-GABARs. (b) Expanded time-base of the traces presented in (a). (*individual event from indicated trace) (c), Averaged values of the coastline length. (d) Averaged ratios of the coastline length (CLL) after L-655 relative to the pre-drug control. n = 12 slices from 6 mice/group.
Blockade of synaptic GABARs only moderately increases spontaneous discharge in high K+ at puberty
We tested the role of synaptic GABARs in modulating epileptiform activity of pubertal hippocampus in high K+ aCSF. To this end, neuronal activity was recorded in pubertal slices before and after bath application of 200 nM SR95531 (gabazine). At this concentration SR95531 exerts an almost complete block of the synaptic GABAR population, selectively4,9. Synaptic GABAR blockade produced a significant reduction in burst amplitude (P = 0.04) but not burst frequency (Table 1). Analysis of coastline length revealed only an 18% increase (pre-SR95531, 10.55 ± 1.5; post-SR95531, 12.5 ± 2.1; P = 0.024, Fig. 5), suggesting that synaptic GABARs have a lesser role in suppressing seizure discharges at puberty than α4βδ GABARs.
Blockade of synaptic GABARs increases spontaneous discharge in high K+ at puberty.
(a) Representative traces of spontaneous synchronized activity recorded in 8.5 mM K+ from pubertal CA1 hippocampus before (left) and after (right) 200 nM SR95531 to block synaptic GABARs. (b) Expanded time-base of the traces presented in (a). (*individual event from indicated trace) (c), Averaged values of the coastline length. (d) Averaged ratios of the coastline length (CLL) after SR95531 relative to the pre-drug control. *(1-tail) paired t-test, t(15) = 2.52, P = 0.024 vs. pre-SR95531, n = 16 slices from 8 mice/group.
Cell-attached recordings of spontaneous activity in high K+ aCSF across pubertal state
In order to determine the activity level of an individual pyramidal cell, we used loose seal cell-attached voltage clamp recordings4 to compare spontaneous activity generated in 8.5 mM K+ in pre-pubertal versus pubertal hippocampus. This technique is advantageous in that it permits assessment of spontaneous activity without disturbing the intracellular milieu. Spiking was decreased by 75% in 8.5 mM K+ at puberty compared to pre-puberty (pre-puberty, 5.3 ± 0.7 spikes/s; puberty, 1.4 ± 0.3 spikes/s; P = 0.00028, Fig. 6), confirming the findings suggested by field recordings that the pubertal hippocampus is less excitable than the pre-pubertal hippocampus in high K+ aCSF.
High K+ triggers less spontaneous discharge at puberty: Cell-attached recordings.
Loose seal, cell-attached recordings from CA1 hippocampal pyramidal cells (taken with the amplifier in voltage clamp mode) reveal a greater rate of spontaneous spiking in 8.5 mM K+ before puberty (Pre-pub, upper trace) compared to puberty (Pub, lower trace). (a) Representative traces. (b) Averaged data. t-test (2-tailed), t(9) = 5.7, *P = 2.8 × 10−4; n = 5–6 mice/group. Inset, current through GABAA receptor-channels is depolarizing in 8.5 mM K+ aCSF. Use of tight-seal, cell-attached recording of a CA1 hippocampal pyramidal cell (taken with the amplifier in current clamp mode) reveals a depolarization of the membrane potential upon application of 5 μM THIP (first arrow) to the slice, which repolarized to baseline upon application of 20 μM bicuculline (second arrow). This depolarization indicates outward Cl− flux. (Representative of 6 cells (1 cell/mouse)).
Determination of the polarity of GABAergic current in high K+ aCSF
Because the desensitization of α4βδ and α5βγ2 GABARs are differentially influenced by the polarity of GABA-gated current19,20, we determined the polarity of the GABAergic current using tight seal, cell-attached current clamp recordings21,22. Previous studies have suggested that high K+ aCSF results in depolarizing GABAergic current, which would selectively accelerate desensitization of α5βγ2 GABARs. For this technique, the use of a >1 GΩ seal and passing 0 current, allows the estimation of the membrane potential change in response to a GABA agonist. Application of the GABA agonist THIP (5 μM) produced an upward deflection (Fig. 6), reflecting outward Cl- flux, that would result in depolarizing GABA current. This deflection returned to baseline upon application of 20 μm bicuculline, verifying the GABAergic nature of the response.
Discussion
This study demonstrates that seizure-like activity generated by a high K+ model is significantly decreased during the pubertal period due to the increased inhibition provided by α4βδ GABARs which emerge during adolescence in CA1 hippocampus4,11. This decrease in epileptiform activity was not seen in the α4−/− mouse hippocampus confirming the role of the α4βδ GABAR in reducing seizure-like activity. This finding may be relevant for remission of childhood epilepsy in adolescence, which is reported for 50–60% of the cases studied2,23.
Patients with epilepsy experience recurrent unprovoked seizures, which are the result of a hypersynchronous discharge of a localized network of pyramidal cells15,24. This seizure discharge would have to last for at least a second or two to result in a clinically-appreciable disturbance, which is why we focused our attention on the presence or absence of long-duration “seizure-like” discharges we observed in our experiments.
In the present study, epileptiform activity was assessed using the coastline length measurement12, which incorporates amplitude, duration and frequency of neuronal activity for a given time period. This parameter was markedly reduced in 8.5 mM KCl at puberty for WT but not α4−/− hippocampus compared to the pre-pubertal hippocampus. In addition, however, the pubertal WT hippocampus displayed virtually no seizure-like activity, compared to the pre-pubertal WT and pubertal α4−/− hippocampi, suggesting that it is more resistant to the expression of seizure activity.
Expression of α4βδ GABARs is increased by up to 8-fold at the onset of puberty in the female mouse CA1 hippocampus compared to nearly undetectable levels pre-pubertally4,11. Expression was established using multiple immunolabeling techniques, including silver-intensified immunogold labeling and electron microscopic quantification, which permits surface localization, while functional expression was confirmed by responses of the tonic current to 100 nM THIP, selective for δ-containing GABARs7,16, using whole cell voltage clamp recording techniques4,10. Our previous studies have shown that the presence of this receptor during the pubertal period (PND 35–44) decreases the input resistance of the neuron and reduces spontaneous activity as well as increasing the threshold for generating an action potential4. In the present study, the reduced excitability of pubertal CA1 neurons was also observed as reduced single cell spiking compared to pre-pubertal hippocampus in the presence of high K+. In all cases, these outcomes were not observed in the α4−/− hippocampus at puberty, confirming the role of the α4βδ GABAR in reducing CA1 pyramidal cell intrinsic excitability at puberty.
GABARs containing the α4 subunit can co-express with γ2 in addition to δ, while δ-containing GABARs can also express with α125. However, at puberty, the majority of receptors which emerge on CA1 hippocampal pyramdiall cells are α4βδ GABARs based on several lines of evidence. First, pubertal pyramidal cells respond robustly to 100 nM THIP, a GABA agonist, which, at this concentration is selective for α4βδ7,16 (rather than α1βδ)26. Secondly, CA1 pyramidal cells of α4−/− mice have significantly reduced surface expression of the GABAR δ subunit and little or no response to 100 nM THIP27, suggesting that knock-out of α4 reduces δ expression to produce a functional α4βδ knock-out. This is not surprising as α1 co-expression with δ has only been reported on interneurons28, suggesting that this sub-type may not express on pyramidal cells. Thus, the recovery of seizure-like activity in the α4−/− is due to the lack of α4βδ GABARs.
Another factor which may have contributed to the pubertal-associated reduction in seizure activity is the neurosteroid THP (3α-OH-5α-pregnan-20-one), a positive GABAR modulator with greatest effects noted at α4βδ GABARs29. Although the day of puberty onset (vaginal opening) is associated with a transient decrease in hippocampal levels of this steroid4, circulating levels of THP would be increased after puberty because it is a metabolite of the ovarian steroid progesterone. THP is known to exhibit anti-convulsant effects30 as a result of its ability to increase GABAergic inhibition. Although our previous findings suggest that THP can exhibit paradoxical effects to reduce inhibition at α4βδ GABARs, these were observed with hyperpolarizing current gated by these receptors4. The depolarizing, inhibitory (shunting) current produced by the high K+ seizure model would be increased by THP and thus would reduce seizure-like activity. We cannot exclude the possibility that other neurotransmitter receptors and ion channels might contribute to the reduced seizure state at puberty, but α4βδ GABARs play a major role because seizure activity was markedly increased in the α4−/− hippocampus.
Other studies have reported increased expression of α4 following pilocarpine injection in a rodent model of temporal lobe epilepsy (TLE)31. Increased expression of α4-containing GABARs and reduced expression of α1-GABARs in this model was associated with increased seizure frequency, which could be reduced by increasing expression of α1-GABARs32. However, in contrast to the present study, this model of TLE also results in reduced δ expression and increased γ2 expression33, suggesting that it increases α4βγ2 GABARs rather than α4βδ GABARs. α4βγ2 GABARs have a faster deactivation34, dependent upon the β isoform and greater desensitization compared to α1βγ234, which would yield less inhibition. For this reason, pubertal reductions in seizure activity may not occur in the dentate gyrus in TLE.
The high K+ seizure model has been used previously to demonstrate seizure-like activity12,13,14, which originates from CA3 hippocampal pyramidal cells triggering seizure bursts in CA1 pyramidal cells via the Schaffer collaterals. Elevated K+ levels occur in seizure states36,37, as well as following increases in neuronal activity38. Increases in extracellular K+ have also been documented to depolarize the membrane and reduce input resistance39, leading to increased hyper-excitability.
The high K+ model is particularly convenient to use to examine developmental changes because it is induced acutely in the slice preparation. Other models would not be feasible for comparing pre-pubertal and pubertal seizure susceptibility because they require a longer time-course to establish the model and thus would overlap both developmental periods.
GABAergic current recorded in the high K+ seizure model at puberty in the present study was a depolarizing, shunting inhibition because depolarization was not sufficient to reach the threshold for triggering an action potential. GABA’s inhibitory effects were observed as a direct reduction in epileptiform activity after bath application of the GABA agonist THIP. However, the current generated by THIP was depolarizing in high K+ aCSF. This outcome was observed as a positive deflection of the voltage recorded using tight-seal cell-attached recordings21. This is in contrast to the typical hyperpolarizing direction of current normally observed in pubertal hippocampus4. The change in polarity observed after exposure to a high K+ solution is likely due to reversal of the direction of the KCC2 K+-Cl− co-transporter which depends upon a K+ gradient40,41. High internal K+ allows for Cl− extrusion, thus maintaining low [Cl−]i which produces hyperpolarizing current in response to GABA. However, this function is reversed in the presence of high external K+. A number of studies have demonstrated that high K+ reduces the ECl− (less negative) and reverses the direction of the transporter, producing depolarizing GABA responses, which are prevented with furosemide, a KCC2 inhibitor42,43,44.
The pubertal reduction in seizure-like activity was largely mediated by α4βδ GABARs selectively. This was confirmed not only by the increase in seizure-like activity after α4 knock-out, but also by the decrease in spontaneous discharge in high K+ produced by δ-selective ligands, THIP and DS2, which did not have significant effects pre-pubertally. Although the effects of these drugs were significant at puberty, they were relatively modest compared to the effect of α4 knock-out. This is likely due to the fact that α4βδ GABARs were close to maximal activation by endogenous GABA, nearly creating a ceiling effect for further anti-convulsant activity by THIP and DS2.
In contrast, blockade of α5β3γ2 GABARs, which account for the majority of extrasynaptic GABARs in CA1 hippocampus8,18, had no significant effect on spontaneous discharge in high K+. This is consistent with other reports demonstrating that L-655,708 is not pro-convulsant at a dose which enhances cognition45. The reason for this difference in effect of the two extrasyaptic GABAR subtypes may be due to the fact that these two receptor sub-types exhibit distinct polarity-dependent increases in their rate of desensitization. When GABA-gated current is depolarizing, as in the high K+ seizure model, α5β3γ2 GABARs have a faster rate of desensitization20, which reduces the steady-state current. The reverse is true for α4βδ GABARs, which desensitize faster and to a greater extent when GABA-gated current is hyperpolarizing19, but yield greater steady-state current when GABA is depolarizing. Thus, in this seizure model, α4βδ GABARs would generate more inhibitory current and provide a more effective anti-convulsant effect.
The polarity-dependent properties of α4βδ GABARs may also be important for certain genetic epilepsies which are due to mutations in α4βδ GABARs that reduce their anti-convulsant effect46. δ-containing GABARs decrease expression in the dentate gyrus in a mouse model of temporal lobe epilepsy induced by pilocarpine, which increases seizure activity33, reflecting the importance of tonic inhibition in reducing seizure activity. However, there are conditions, such as brain trauma, in which GABAergic current becomes depolarizing and excitatory47. This outcome would be resistant to the anti-convulsant effects conferred by pubertal expression of α4βδ GABARs.
In contrast to the pronounced effect of α4 knock-out, blockade of the synaptic GABARs had a relatively minor effect on seizure activity (18% versus >100% produced by α4 knock-out) suggesting that tonic inhibition generated by α4βδ GABARs is the primary anti-convulsant mechanism at puberty. This is consistent with reports suggesting that the inhibition produced by the tonic current is greater than that produced by phasic currents48.
Remission for childhood epilepsy is currently reported for over 60% of children studied2,23, an effect which is not gender-specific, suggesting that this is a robust outcome. It is most common in children with an onset of first seizure <10 years of age and a mean age of remission around the time of puberty onset2, consistent with the results from the present study. There is a greater probability of remission for uncomplicated, idiopathic seizures which have a frequency of less than 1 seizure/week, unaccompanied by intellectual disability or EEG changes23, which are less likely to involve neurodegeneration which may indicate a permanent change in network properties. To date there is no physiological explanation for this finding. Although the most common epilepsies to remit are the primary generalized epilepsies, which do not originate in the temporal lobe, this may be due to the decrease in δ expression in dentate gyrus reported in TLE33. Increased δ expression has also been observed in the adolescent cortex49 in addition to CA1 hippocampus10 suggesting that the anti-convulsant actions of α4βδ GABARs at puberty are site-specific. The present findings suggest a possible mechanism for remission of certain childhood epilepsies and may also suggest possible therapeutic interventions for those epilepsies which do not undergo remission in adolescence.
Methods
Animals
Pre-pubertal (~PND 26–32) or pubertal (~PND 35–44) female C57BL6 mice (wild-type (WT) or α4−/−) were used. α4−/− mice were bred from α4+/− (generously supplied by G. Homanics, U. Pittsburgh). Initially, +/+ mice were used, but supplemented with wild-type mice from Jackson labs because they produced similar outcomes to the +/+ mice bred in house. Female mice were used because α4βδ GABAR expression and physiological effects have been well characterized across pubertal development4,10. Animals were housed in a reverse light:dark cycle vivarium and tested 1 h before the onset of the dark cycle. Puberty onset was verified by vaginal opening4. The estrous cycle does not alter GABAergic inhibition during the pubertal period, as we have previously shown4. All procedures were approved by and carried out in accordance with the Institutional Animal Care and Use Committee at SUNY Downstate.
Hippocampal slice preparation
Animals were rapidly decapitated and the brains removed and cooled using an ice cold solution of artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl 124, KCl 5, CaCl2 2, KH2PO4 1.25, MgSO4 2, NaHCO3 26 and glucose 10, saturated with 95% O2, 5% CO2 and buffered to a pH of 7.4. The hippocampus was removed and 400–450 μm transverse sections were prepared using a Leica oscillating microtome (cell attached recordings) or a McIlwain tissue chopper (extracellular recordings); slices were then incubated for 1 h in oxygenated aCSF (95% O2 and 5% CO2). Data were collected from 1–2 slices/animal.
Electrophysiology-extracellular field recordings
The slices were initially perfused in an interface configuration for >1 h as the bath temperature was raised to 30 ± 1 °C. During the recording period, the submerged configuration was used to permit optimal solution exchange. The control aCSF contained (in mM): NaCl 124, KCl 5, NaH2PO4 1.25, NaHCO3 26, CaCl2 2, MgCl2 1.6 and glucose 10 (pH 7.4).
Extracellular recordings were made from the CA1 pyramidal cell body layer using borosilicate glass pipets filled with aCSF (resistance 2–3 MΩ). Spontaneous activity was recorded with AxoScope (MiniDigi 1B, pClamp 10.1, 1 kHz sampling rate) after continuous bath perfusion of 8.5 mM KCl for at least 1 h to elicit a stable pattern of epileptiform activity12. For some experiments, GABAR subunit selective drugs were bath applied following stable baseline recordings in the presence of 8.5 mM KCl and spontaneous activity recorded.
Electrophysiology-cell attached
Pyramidal cells in CA1 hippocampal slice were visualized using a Leica differential interference contrast (DIC)-infrared upright microscope. Recordings were carried out at 22–24 °C using an Axopatch 200B amplifier, at a 10-kHz sampling frequency (2 kHz 4-pole Bessel filter) and pClamp 9.2 software (Molecular Devices, Sunnyvale, CA). Patch pipets were fabricated from borosilicate glass using a Flaming-Brown puller (Sutter Instruments, Novato, CA, USA) to yield open tip resistances of 2–4 MΩ. The bath contained (in mM): NaCl 124, KCl 5, CaCl2 2, KH2PO4 1.25, MgSO4 2, NaHCO3 26 and glucose 10, saturated with 95% O2/5% CO2 and buffered to a pH of 7.4.
Cell-attached recordings of pyramidal cell spiking
In some cases, loose seal cell-attached recordings were made with the amplifier in voltage clamp mode to assess cell spiking before and after bath application of 8.5 mM KCl, used as a seizure model. The pipet solution contained 150 mM NaCl. The command potential was set to the potential at which the holding current was 0 pA to avoid direct cell stimulation by the electrode20. This technique is advantageous in permitting the evaluation of neuronal excitability without disturbing the internal Cl− milieu4.
Cell-attached recording of polarity of GABAA receptor-channel-mediated potential
We used tight-seal cell-attached recording techniques with the amplifier in current clamp mode21,22 to assess whether the GABA agonist THIP yielded a hyperpolarizing or depolarizing potential in hippocampal CA1 pyramidal cells in slices from pre-pubertal vs. pubertal mice. With a >1 GΩ seal and passing 0 current, Rseal ≫ Rpatch+cell allows determination of the direction of potential change in response to GABA20 or in response to an exogenously-applied GABA agonist, as we have previously demonstrated4. Therefore, for this study, tight-seal (>1 GΩ) cell-attached recording of membrane potentials were made from the soma of CA1 hippocampal pyramidal cells. The pipet solution contained 150 mM NaCl. The response of the membrane potential to bath application of 5 μM THIP was recorded (with no current injected). For this study, the bath contained tetrodotoxin (TTX, 0.5 μM) to isolate the post-synaptic component and 2 mM kynurenic acid to pharmacologically isolate the GABAergic current.
Drugs
For some experiments, GABAR subunit selective agents were bath applied following stable baseline recordings in the presence of 8.5 mM KCl in order to determine potential mechanisms for pubertal decreases in seizure-like activity. These agents include: DS2 (4-Chloro-N-[2-(2-thienyl)imidazo[1,2-a]pyridin-3-yl]benzamide, 10 μM), a positive GABAR modulator selective for α4βδ GABARs50, THIP (gaboxadol or 4,5,6,7-tetrahydroisoxazolopyridin-3-ol, 1 μM), a GABAR agonist selective for α4βδ GABARs at 1 μM7,16, L-655,708 (11,12,13,13a-Tetrahydro-7-methoxy-9-oxo-9H-imidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylic acid, ethyl ester, 50 nM), an inverse agonist at α5βγ2 GABARs18 and SR-95531 (gabazine or 6-Imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide), a GABA antagonist which selectively blocks the phasic current up tp 90% at 200 nM4,9. All GABAR agents were obtained from Tocris Bioscience. All other chemicals were from Sigma Chemical Co.
Data analysis
Spontaneous field potentials were detected using a threshold delimited event detection program in pClamp 10.1. In extracellular field recordings of spontaneous discharge, an event was defined as a deflection >0.025 mV from baseline which recovered to baseline. Seizure-like (“ictal”) discharges were defined as synchronous, large amplitude (>2 mV), high frequency (>2/s) events of >2 s duration15. Slices which did not exhibit seizure-like activity in 8.5 mM K+ displayed synchronized discharge with reduced frequency, amplitude and duration (“inter-ictal” activity) compared to the seizure-like activity described above.
Epileptiform activities recorded from field recordings are irregular and difficult to quantify by conventional methods; therefore, the coastline length (CLL)12 was calculated because it more accurately reflects the strength of such activity. CLL is the summary of the point-to-point distance of a given segment (e.g, 10 min), which defines the overall intensity of spontaneous, synchronized discharge incorporating amplitude and frequency (minus the noise). The MiniAnalysis program (Synaptosoft, Inc., Decatur, GA) was used to calculate the CLL to compare neuronal activity across groups. In cases where drugs were applied directly to the slice, frequency and peak amplitude measures of field potentials were also calculated before and after drug application.
Clampfit was used to analyze the frequency and amplitude of the epileptiform activity as well as to determine the direction of GABAergic current and calculate spike frequency produced by high K+ in cell-attached recordings.
Statistics
Origin (OriginLab, Northampton, MA) was used for all statistical comparisons. Statistical differences among 3 groups were determined using an analysis of variance (ANOVA), followed by post-hoc Tukey’s tests; differences between 2 groups were determined using the Student’s t test or the paired t-test when comparing discharge before and after drug application in the same recording. Differences in the percentage of slices with seizure-like activity were determined using a Chi-square analysis (3 × 2contingency table). Data were shown to fit a normal distribution using the Kolmogorov-Smirnov test for normality. A P < 0.05 was considered as statistically significant. All data are described as the mean ± S.E.M. A description of the statistics used for each experiment is included in the figure legends.
Additional Information
How to cite this article: Yang, L. et al. Pubertal Expression of α4βδ GABAA Receptors Reduces Seizure-Like Discharges in CA1 Hippocampus. Sci. Rep. 6, 31928; doi: 10.1038/srep31928 (2016).
References
Neville, B. G., Chin, R. F. & Scott, R. C. Childhood convulsive status epilepticus: epidemiology, management and outcome. Acta Neurol Scand Suppl 186, 21–24 (2007).
Berg, A. T., Rychlik, K., Levy, S. R. & Testa, F. M. Complete remission of childhood-onset epilepsy: stability and prediction over two decades. Brain 137, 3213–3222, doi: 10.1093/brain/awu294 (2014).
Camfield, C. S., Berg, A., Stephani, U. & Wirrell, E. C. Transition issues for benign epilepsy with centrotemporal spikes, nonlesional focal epilepsy in otherwise normal children, childhood absence epilepsy and juvenile myoclonic epilepsy. Epilepsia 55 Suppl 3, 16–20, doi: 10.1111/epi.12706 (2014).
Shen, H. et al. Reversal of neurosteroid effects at alpha4-beta2-delta GABA-A receptors triggers anxiety at puberty. Nat Neurosci 10, 469–477 (2007).
Olsen, R. W. & Sieghart, W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56, 141–148, doi: 10.1016/j.neuropharm.2008.07.045 (2009).
Stell, B. M., Brickley, S. G., Tang, C. Y., Farrant, M. & Mody, I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA-A receptors. Proc Natl Acad Sci 100, 14439–14444 (2003).
Brown, N., Kerby, J., Bonnert, T. P., Whiting, P. J. & Wafford, K. A. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br. J. Pharmacol 136, 965–974 (2002).
Wisden, W., Laurie, D. J., Monyer, H. & Seeburg, P. The distribution of 13 GABA-A receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci 12, 1040–1062 (1992).
Stell, B. M. & Mody, I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J. Neurosci. 22, RC223 (2002).
Shen, H. et al. A critical role for alpha4betadelta GABAA receptors in shaping learning deficits at puberty in mice. Science 327, 1515–1518, doi: 10.1126/science.1184245 (2010).
Shen, H. et al. A critical role for alpha4-beta-delta GABA-A receptors in shaping learning deficits at puberty in mice. Science (Supp.Online Material) 327, 1515–1518, doi: 327/5972/1515 [pii];10.1126/science.1184245 (2010).
Traynelis, S. F. & Dingledine, R. Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J Neurophysiol 59, 259–276 (1988).
Korn, S. J., Giacchino, J. L., Chamberlin, N. L. & Dingledine, R. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. J Neurophysiol 57, 325–340 (1987).
Jensen, M. S. & Yaari, Y. Role of intrinsic burst firing, potassium accumulation and electrical coupling in the elevated potassium model of hippocampal epilepsy. J Neurophysiol 77, 1224–1233 (1997).
Merlin, L. R. Responsiveness of ictaform discharges to pharmacotherapy: the bigger they are, the harder they fall. Epilepsy Currents / American Epilepsy Society 10, 102–104, doi: 10.1111/j.1535-7511.2010.01371.x (2010).
Meera, P., M, W. & Otis, T. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABA-A receptors. J.Neurophysiology 106, 2057–2011 (2011).
Wafford, K. A. et al. Novel compounds selectively enhance delta subunit containing GABA A receptors and increase tonic currents in thalamus. Neuropharmacology 56, 182–189, doi: S0028-3908(08)00346-8 [pii];10.1016/j.neuropharm.2008.08.004 (2009).
Caraiscos, V. B. et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha 5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci 101, 3662–3667 (2004).
Bianchi, M. T., Haas, K. F. & Macdonald, R. L. Alpha1 and alpha6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABA(A) receptors containing the delta subunit. Neuropharm. 43, 492–502 (2002).
Burgard, E. C., Tietz, E. I., Neelands, T. R. & Macdonald, R. L. Properties of recombinant gamma-aminobutyric acid A receptor isoforms containing the alpha 5 subunit subtype. Mol Pharmacol 50, 119–127 (1996).
Perkins, K. L. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J Neurosci Methods 154, 1–18, doi: 10.1016/j.jneumeth.2006.02.010 (2006).
Mason, M. J., Simpson, A. K., Mahaut-Smith, M. P. & Robinson, H. P. The interpretation of current-clamp recordings in the cell-attached patch-clamp configuration. Biophys J 88, 739–750 (2005).
Sillanpaa, M., Saarinen, M. & Schmidt, D. Clinical conditions of long-term cure in childhood-onset epilepsy: a 45-year follow-up study. Epilepsy Behav 37, 49–53, doi: 10.1016/j.yebeh.2014.05.029 (2014).
D’Antuono, M. et al. Antiepileptic drugs abolish ictal but not interictal epileptiform discharges in vitro. Epilepsia 51, 423–431, doi: 10.1111/j.1528-1167.2009.02273.x (2010).
Sur, C. et al. In Mol Pharmacol 56, 110–115 (1999).
Jia, F. et al. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol 94, 4491–4501, doi: 10.1152/jn.00421.2005 (2005).
Sabaliauskas, N., Shen, H., Homanics, G. E., Smith, S. S. & Aoki, C. Knockout of the gamma-aminobutyric acid receptor subunit alpha4 reduces functional delta-containing extrasynaptic receptors in hippocampal pyramidal cells at the onset of puberty. Brain Res 1450, 11–23, doi: S0006-8993(12)00312-5 [pii];10.1016/j.brainres.2012.02.035 (2012).
Glykys, J. et al. A new naturally occurring GABA-A receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci 10, 40–48 (2007).
Wohlfarth, K. M., Bianchi, M. T. & Macdonald, R. L. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J. Neurosci. 22, 1541–1549 (2002).
Frye, C. A. The neurosteroid 3 alpha, 5 apha-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res 696, 113–120 (1995).
Grabenstatter, H. L. et al. Effect of spontaneous seizures on GABAA receptor alpha4 subunit expression in an animal model of temporal lobe epilepsy. Epilepsia 55, 1826–1833, doi: 10.1111/epi.12771 (2014).
Raol, Y. H. et al. Enhancing GABA(A) receptor alpha 1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J Neurosci 26, 11342–11346, doi: 10.1523/JNEUROSCI.3329-06.2006 (2006).
Peng, Z., Huang, C. S., Stell, B. M., Mody, I. & Houser, C. R. Altered expression of the delta subunit of the GABA-A receptor in a mouse model of temporal lobe epilepsy. J.Neurosci 24, 8629–8639 (2004).
Smith, S. S. & Gong, Q. H. Neurosteroid administration and withdrawal alter GABA-A receptor kinetics in CA1 hippocampus of female rats. J Physiol 564, 421–436 (2005).
Lagrange, A. H., Botzolakis, E. J. & Macdonald, R. L. Enhanced macroscopic desensitization shapes the response of alpha4 subtype-containing GABAA receptors to synaptic and extrasynaptic GABA. J Physiol 578, 655–676, doi:10.1113/jphysiol.2006.122135 (2007).
Pedley, T. A., Fisher, R. S., Moody, W. J., Futamachi, K. J. & Prince, D. A. Extracellular potassium activity during epileptogenesis: a comparison between neocortex and hippocampus. Trans Am Neurol Assoc 99, 41–45 (1974).
Fisher, R. S., Pedley, T. A., Moody, W. J., Jr. & Prince, D. A. The role of extracellular potassium in hippocampal epilepsy. Arch Neurol 33, 76–83 (1976).
Frohlich, F., Bazhenov, M., Iragui-Madoz, V. & Sejnowski, T. J. Potassium dynamics in the epileptic cortex: new insights on an old topic. Neuroscientist 14, 422–433, doi: 10.1177/1073858408317955 (2008).
Kaila, K., Lamsa, K., Smirnov, S., Taira, T. & Voipio, J. Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J Neurosci 17, 7662–7672 (1997).
Payne, J. A. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am J. Physiol 273, C1516–C1525 (1997).
Payne, J. A., Rivera, C., Voipio, J. & Kaila, K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci 26, 199–206 (2003).
Thompson, S. M. & Gahwiler, B. H. In J. Neurophysiol. 61, 512–523 (1989).
Jensen, M. S., Cherubini, E. & Yaari, Y. Opponent effects of potassium on GABAA-mediated postsynaptic inhibition in the rat hippocampus. J Neurophysiol 69, 764–771 (1993).
DeFazio, R. A., Keros, S., Quick, M. W. & Hablitz, J. J. In The Journal of Neuroscience 20, 8069–8076 (2000).
Atack, J. R. et al. L-655,708 enhances cognition in rats but is not pro-convulsant at a dose selective for alpha5-containing GABAA receptors. Neuropharm 51, 1023–1029 (2006).
Feng, H. J. et al. Delta subunit susceptibility variants E177A and R220H associated with complex epilepsy alter channel gating and surface expression of alpha4beta2delta GABAA receptors. J Neurosci 26, 1499–1506, doi: 10.1523/JNEUROSCI.2913-05.2006 (2006).
Dzhala, V. & Staley, K. J. Acute and chronic efficacy of bumetanide in an in vitro model of posttraumatic epileptogenesis. CNS neuroscience & therapeutics 21, 173–180, doi: 10.1111/cns.12369 (2015).
Bai, D. et al. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by y-aminobutyric acid(A) receptors in hippocampal neurons. Molec Pharmac 59, 814–824 (2000).
Datta, D., Arion, D. & Lewis, D. A. Developmental Expression Patterns of GABAA Receptor Subunits in Layer 3 and 5 Pyramidal Cells of Monkey Prefrontal Cortex. Cereb Cortex 25, 2295–2305, doi: 10.1093/cercor/bhu040 (2015).
Wafford, K. A. et al. Novel compounds selectively enhance delta subunit containing GABA A receptors and increase tonic currents in thalamus. Neuropharmacology 56, 182–189, doi: 10.1016/j.neuropharm.2008.08.004 (2009).
Acknowledgements
We thank G. Homanics (Univ. Pittsburgh) for supplying the α4+/− mice. We also thank Chen Zheng (Tianjin Medical Univ.) and Yuanyuan Li (Tianjin Medical Univ.) for helpful technical assistance. This work was supported by R01-MH100561 to S.S.S.
Author information
Authors and Affiliations
Contributions
L.Y. contributed to the experimental design, performed extracellular recordings and analyzed data; H.S. performed cell-attached recordings; L.R.M. contributed to the experimental design and writing of the paper; S.S.S. designed the experiments, analyzed data, constructed the figures and wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yang, L., Shen, H., Merlin, L. et al. Pubertal Expression of α4βδ GABAA Receptors Reduces Seizure-Like Discharges in CA1 Hippocampus. Sci Rep 6, 31928 (2016). https://doi.org/10.1038/srep31928
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep31928
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.