Abstract
Research has shown that distinct insular subregions are associated with particular neural networks (e.g., attentional and sensorimotor networks). Based on the evidence that playing action video games (AVGs) facilitates attentional and sensorimotor functions, this study examined the relation between AVG experience and the plasticity of insular subregions and the functional networks therein that are related to attentional and sensorimotor functions. By comparing AVG experts and amateurs, we found that AVG experts had enhanced functional connectivity and grey matter volume in insular subregions. Furthermore, AVG experts exhibited increased functional connectivity between the attentional and sensorimotor networks and the experience-related enhancement was predominantly evident in the left insula, an understudied brain area. Thus, AVG playing may enhance functional integration of insular subregions and the pertinent networks therein.
Similar content being viewed by others
Introduction
Action video games (AVGs) are becoming increasingly popular worldwide. Similar to conventional sports (e.g., basketball, tennis), AVG playing requires a high level of attention and hand-eye coordination (read Supplemental materials for an example of an AVG). Given its influence on the development of cognitive functions, AVG experience has attracted increasing research attention recently1.
Behavioural research has shown that experienced AVG players have better attentional and sensorimotor functions than amateurs. For example, compared to amateurs, AVG experts exhibited improved selective attention on tasks of flanker compatibility, enumeration, useful field of view and attentional blink; furthermore, AVG training improved participants' performance on the above tasks, thereby demonstrating the attentional effects of AVG playing2. Furthermore, AVG playing enhanced the spatial distribution of attention and attentional capture3,4, cognitive control5 and emotional regulation6. In addition, research on sensorimotor functions indicated that compared to amateurs, AVG experts had improved spatial resolution of vision7, multisensory temporal processing abilities8, hand-eye motor coordination9, contrast sensitivity10, oculomotor performance11 and body movement12.
Neuroscience research has also examined the neural basis of cognitive benefits of AVG experience. For example, AVG playing can change cortical networks for complex visuomotor transformation13, reduce the amplitude of visually evoked potentials during a non-voluntary attention condition14 and modulate P2 and P3 evoked potentials15. Furthermore, AVG experience is also associated with increased grey matter volume (GMV) in the dorsal striatum16,17, right posterior parietal cortex18, entorhinal cortex, hippocampus, occipital cortex19, right hippocampal formation and right dorsolateral prefrontal cortex, as well as in both hemispheres of the cerebellum20.
However, little research has examined the relation between AVG experience and the plasticity of insula, an important brain area for attentional and sensorimotor functions. Insula contains multiple subregions and is related to various functions21. By examining resting-state functional connectivity (FC, which reflects the interaction among neuronal populations), Cauda et al. found that 1) the anterior insular subregions are involved in an anterior attentive network (A-network), which includes the superior, middle and inferior frontal gyri, the temporoparietal junction, the rostral anterior cingulate cortex, the cuneus, the precuneus and the superior temporal gyri; 2) the posterior subregions are involved in a posterior sensorimotor network (P-network), which includes the sensorimotor, supplementary motor, superior temporal, middle temporal, lingual and cerebellar cortices; and 3) a transitional subregion between the anterior and the posterior subregions22. Furthermore, the proposition that the anterior and posterior subregions are related to the A- and P-networks respectively was supported by probabilistic tract-tracing research23, meta-analyses24 and primate data9,25.
This study examined AVG-related effects on the plasticity of insular subregions and functional networks therein. We hypothesized that AVG experience is associated with an enhancement of insular subregions and A- and P-networks. This hypothesis was based on two aforementioned findings: AVG playing enhances both attentional and sensorimotor functions; and insular subregions and their functional networks play a crucial role in attentional and sensorimotor functions. Additionally, studies on neuroplasticity indicated that the FC of brain develops with age26 and can be adapted by learning activities27; furthermore, gaining experience can induce an increase of GMV in adults28 and the elderly29. We therefore used FC and GMV as measurements of insular function and structure, respectively.
To evaluate our hypothesis, we tested two groups of subjects: AVG experts and amateurs. The AVG experts were highly experienced AVG players who had at least 6 years of AVG experience and were recognised as regional or national champions. The amateurs did not play AVG habitually and had less than one year of AVG experience. We examined 1) the FC and GMV of ten bilateral subregions, 2) the FC of the A-and P-networks and 3) the correlations among FC, GMV and average weekly amount of time participants spent playing AVG (AT).
Enhanced FC of insular subregions
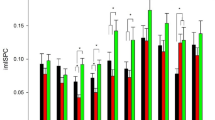
Compared with amateurs, AVG experts showed significantly higher FC between insular subregions. Furthermore, AVG experts with higher FC also showed anterior-posterior integration and left-lateralisation (Figure 1). The pattern of results is confirmed by insular functional integration analysis (described in Data Analysis), which showed greater insular functional integration in AVG experts than in amateurs [left: t(55) = 2.93, right: t(55) = 2.73, p's <0.009]. Correlational analyses showed that only the left insular functional integration in experts was correlated with their AT (Figure 2a).
Enhanced FC of the insular subregions.
The white lines denote the pathways where experts had significant enhancements compared with amateurs, p <0.05, FDR-corrected. The dashed line represents p <0.01, uncorrected. The higher FC reveals a pattern of anterior-posterior integration and left-lateralisation. ROIs 1, 2, 4, 5 and 8 (green) were located in the anterior subregions. ROIs 3, 7 and 10 (red) were located in the posterior subregions. ROIs 6 and 9 (yellow) were located in the transitional subregions.
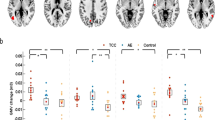
Correlation and GMV subregion analysis.
2a, 2b and 2c are pairwise Pearson correlations that include insular functional integration, GMV and the average playing time. 2d is a comparison between the two groups within the left long insular gyrus and central sulcus. 2e illustrates an inflated surface of the left hemisphere. The long insular gyrus and central sulcus is shown in grey and is located near the transitional subregion.
Increased GMV of insular subregions
In line with the FC subregion analysis, a significant increase in GMV was observed in the left insula in experts (Figures 2b). Further analyses revealed an increased GMV in the short insular gyri, long insular gyrus and central sulcus (Figures 2c). Correlational analyses showed that the increase of GMV in long insular gyrus and central sulcus of the left hemisphere were correlated with insular functional integration and AT in experts (Figures 2d). Correlational analyses in the amateur group did not reveal significant results.
Enhanced FC of networks
In most ROIs, the amateurs' FC networks in this study were similar to their counterparts' in Cauda et el. (2010). ROIs 1, 2, 4, 5 and 8 showed a bilateral pattern (A-network) involving the superior, middle and inferior frontal gyri, as well as the bilateral temporoparietal junction, anterior cingulate, superior temporal gyri and putamen (Figure 3a); ROIs 3, 7 and 10 also showed a bilateral pattern (P-network) linking the precentral gyri, postcentral gyri, superior temporal gyri, middle temporal gyri, supplement motor area, etc. (Figure 3b); and ROIs 6 and 9 exhibited the transition between the A- and P-networks (FC networks of all ROIs are presented in Supplemental Figure 1).
Examples of the A- and P-networks.
The left ROI 4 is shown as a representative example in 3a and 3c and the left ROI 10 is shown as a representative example in 3b and 3d. A spatial distribution similar to the left ROI 4 was observed in the other anterior ROIs and a spatial distribution similar to the left ROI 10 was observed in the other posterior ROIs. See Supplemental Figure 1 for the analysis of other ROIs. Colours ranging from green to yellow or red to yellow indicate increasing spatial consistency (%). E.g., a 40% value in the amateur group indicates that the relevant brain region was activated in 12 subjects [40% = (12/30) × 100%]. The blue circle indicates the MFG, which is believed to be a key node in the A-network and was also identified in the P-networks of experts. The maps are projected onto a 3D brain surface using the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
Furthermore, we found a similar pattern of results in experts and amateurs in the anterior ROIs (Figure 3c) but not in the posterior ROIs (Figure 3d). In AVG experts, the ROIs within the posterior subregions were associated with the bilateral middle frontal gyrus (MFG), which was confirmed by the analysis of spatial consistency based on probability networks. The MFG is widely accepted as a key node in the A-network (blue arrow in Figure 3d). In addition, FC was enhanced within the A- and P-networks in the left vs. right insula of experts (Figure 4). See Supplemental Tables 1 and 2 for details.
Discussion
Previous studies have shown that AVG playing is associated with improved performance on tasks that demand attention and/or sensorimotor abilities (e.g., AVG experts exhibit better visual selective attention, visuospatial attention, multisensory temporal processing abilities and hand-eye motor coordination). Furthermore, the anterior and posterior subregions of the insula are involved in an attentional network (A-network) and a sensorimotor network (P-network), respectively.
Enhanced functional integration of subregions
This study found enhanced insular functional integration between the anterior and posterior subregions of the experts, predominately in the left insula (refer to Figure 1). The finding that left insular functional integration was correlated with AT in experts suggests an AVG-related enhancement21. Since the anterior and posterior subregions are involved in attentional and sensorimotor functions respectively22, the enhancement observed in experts is consistent with the previous finding that attention is essential for sensorimotor functions30. This enhancement may serve as the neural basis of the improved coordination between attentional and sensorimotor functions in experts.
Increased GMV in insular subregions
Longitudinal studies have revealed that acquiring knowledge31 and learning skills28 can enhance the activity of relevant brain areas. Accordingly, researchers proposed that the increased GMV in insula is induced by relevant learning activities. For example, Giuliani et al. found that the use of expressive suppression can predict the GMV of the anterior subregions32 and that the neural activity of the left anterior subregions can be modulated by voluntary regulation in a real-time manner33; patients with better stroke recovery exhibit greater activation of the insula34; insular enhancement is related to learning languages35 and music36; and the GMV of the left posterior insular subregions is related to compensatory sensorimotor function in the deaf, who rely more on visual-motoric representations than the normal hearing37. Converging neuroscience evidence therefore suggests that the insula, particularly the left side, is sensitive to certain learning activities. Given the importance of attentional and sensorimotor functions in learning21, it is highly likely that the insula is enhanced by long-term AVG playing. The present study supports the above speculation by revealing increased GMV in the left long insular gyrus and central insular sulcus (Figure 2b). Further evidence demonstrated that only the GMV of the left long insular gyrus and central insular sulcus was correlated with insular functional integration and AT in the experts (Figures 2a and 2b).
Why is the long insular gyrus and central insular sulcus related to insular functional integration? This region is located near the transitional subregion (Figures 1 and 2e)19 between the anterior and the posterior subregions. Thus, the increased GMV of the long insular gyrus and central insular sulcus might enhance the functional integration between the anterior and posterior subregions. However, this is merely a conjecture yet to be tested by experimental studies.
Enhanced functional integration in the insular networks
Can the enhancement in the insular subregions influence the functional networks therein? This study suggests that it can, based on the results of two comparisons: amateurs in this study vs. Cauda et al.'s study and anterior and posterior subregions in amateurs vs. experts.
First, we found a pattern of results similar to Cauda et al., such that the anterior and posterior subregions of amateurs contained distinct networks (Figures 3a and 3b). This finding supports Cauda et al.'s proposition that the anterior and posterior subregions are involved in the A- and P-networks respectively, which is also in accordance with previous findings on WM23, active task24 and anatomy38.
Second, we found a similar pattern of results in experts and amateurs in anterior subregions (Figure 3c), such that the enhanced FC had no effects on the A-network. However, we found significant differences between experts' and amateurs' posterior ROIs (Figure 3d). The bilateral MFGs were linked to posterior ROIs in experts, while they tended to be linked to anterior ROIs in amateurs. The finding was confirmed by a spatial consistency analysis (Figure 3d). The results suggested that enhanced FC in the subregions might influence the P-network pattern in experts. Given the results of GMV and FC of the subregions, we propose that the activity observed in the MFG in experts may indicate an enhanced functional integration between A- and P-networks. Furthermore, the subregional adaptation may serve as the neural basis of the functional integration between A- and P-networks.
In addition, although the MFG is usually classified as a node of the A-network22,23,39,40, evidence has shown that the MFG is involved in control networks and is activated in higher-level functions, such as attention, spatial and episodic memory and explicit contingency awareness41,42,43,44. The MFG should also be involved in the sensorimotor network even in amateurs45. However, studies have not found significant FC between the MFG and the sensorimotor networks in amateurs, which is perhaps due to the fact that the path length between the MFG and the sensorimotor networks is greater in amateurs than in experts46. Thus, the intermediate brain area, which connects the MFG and the sensorimotor networks, may decrease the possibility of detecting significant FC. Here, path length refers to the distance from one node to another in a network. A shorter path reflects fewer intermediate areas and indicates an increased interactive efficiency between nodes47. See Figure 5 for a summary of the findings of this study.
A summary of the findings of this study.
The shapes represent brain areas (MFG, insula and a putative intermediate brain area). The lines with two arrowheads represent FC. 5a corresponds to the pattern of the insular network of the amateurs in this study and in Cauda et al.'s study 5b corresponds to the pattern observed in the insular network of the experts. Note that 5b shows an expanded transitional subregion (the dotted line circle) and a direct connection between the MFG and posterior subregion (purple line).
Why does a AVG expert's insula surpass an amateur's
Attention is a determinant mechanism in sensorimotor functions48. In a typical AVG game, players may complete approximately 150 sensorimotor responses per minute using the keyboard and mouse, which requires coordination between attention and sensorimotor functions. As aforementioned, the anterior and posterior insular subregions are involved in attentive and sensorimotor networks respectively. AVG playing therefore demands for integration between attentive and sensorimotor networks, which may induce neural plasticity in the insula.
Conclusion
By comparing AVG experts and amateurs, this study found enhancements in the AVG experts' FC between anterior and posterior insular subregions, GMV in the long insular gyrus and central insular sulcus and functional integration between the attentional and sensorimotor networks. Furthermore, experience-based enhancement was predominately evident in the left insula. These results suggest that AVG playing may induce functional integration of insular subregions and pertinent networks therein. Currently, a longitudinal experimental study is examining the causal relation between AVG playing and neuroplasticity.
Methods
Subjects
The study protocol was approved by the ethics research committee at the University of Electronic Science and Technology of China (UESTC) and has been performed in accordance with ethical standards outlined by the Declaration of Helsinki. Informed consent was obtained from all subjects. A total of 27 AVG experts (mean age = 23.26 ± 0.4 years) and 30 amateurs (22.3 ± 0.38 years) participated in this study. All of the subjects were right-handed according to the Edinburgh Inventory49, reported normal or corrected-to-normal vision, had normal hearing and reported no history of neurological illnesses.
The experts were highly experienced players of AVGs (i.e., League of Legends [LOL] or Defence of the Ancient 2 [DOTA 2]). They had received AVG training for at least six years and were recognized as either regional or national champions in AVG competitions. The amateurs did not play AVG habitually and had less than one-year AVG experience. The experts' AVG experience was quantified based on their professional score, ranging from 1900 to 2600 points, measured on Elo's chess-skill rating scale50. The amateurs had less than 1200 points. The Elo rating scale is widely used as a rating system for multiplayer competition in AVGs. The difference in the ratings between two players serves as a predictor of the outcome of a match. A difference of 100 points indicates that the probability of winning a AVG match for the stronger player is 64%, 200 points is 76%. In general, an AVG expert has a rating of 1800 points or higher, while an amateur has a rating of approximately 1200 points.
Confounding variables were matched between groups (see Table 1, also see Du et al., 2011)51. The only significant between-group differences were average weekly amount of time spent on AVG Playing (AT) and game type (t = 12.39, χ2 = 20.2 p's <0.0001), which further verified the group membership (i.e., experts vs. amateurs). Among the various behavioral measurements we used to gauge the experts' AVG experience, the experts' AT, appeared to be the most sensitive indicator to their AVG capability, since the experts' professional scores and performance on Dodge The Squares were correlated with their AT (rscores = 0.41, rperformance = 0.45, ps <0.03).
Data acquisition
Images were acquired on a 3T MRI scanner (GE Discovery MR750) at the MRI research centre of UESTC. Resting state functional MRI data were acquired using gradient-echo EPI sequences (repetition time [TR] = 2000 msec, echo time [TE] = 30 msec, flap angle [FA] = 90°, matrix = 64 × 64, 3 × 3 × 3 mm voxels, field of view [FOV] = 24 × 24 cm2, slice thickness/gap = 4 mm/0.4 mm), with an eight channel-phased array head coil. All of the subjects underwent a 510 sec resting state scan that yielded 255 volumes (32 slices per volume). High-resolution T1-weighted images were acquired using a 3-dimensional fast spoiled gradient echo (T1-3D FSPGR) sequence (TR = 6.008 msec, TE = 1.984 msec, FA = 9°, matrix = 256 × 256, FOV = 25.6 × 20 cm2 [80%], slice thickness [no gap] = 1 mm) to generate 152 slices.
Data analysis
Data analysis was divided into two sections, FC analysis and GMV analysis. The FC analysis implicated both subregions and functional networks and used an identical data pre-processing procedure. The FC subregion analysis was computed on pair-wise subregions, whereas the FC functional network analysis was conducted between subregions and voxels outside of the insula. The GMV analysis was performed only to examine subregions of interest.
Functional MRI data pre-processing
Functional MRI data pre-processing followed typical pre-processing procedures using SPM8 (Wellcome Department of Cognitive Neurology, London, UK) and customised Matlab scripts. These procedures included discarding the first five volumes of each run, slice scan time correction, head motion correction52 and image normalisation using an EPI template from the Montreal Neurological Institute (MNI) atlas space. Spatial smoothing was applied with a Gaussian kernel of 8 mm full-width half-maximum (FWHM). Temporal filtering (band-pass) was between 0.01–0.08 Hz and the mean signal was removed.
Defining regions of interest (ROIs)
To avoid preconceptions during the segmentation of subregions, we selected the coordinates used by Cauda et al. (2011). Following this publication, we defined our ROIs in an equispaced pattern. In short, twenty ROIs (left insula: 1–10, right insula: 1–10) were chosen in three horizontal planes that were left-right identical (Figure 1). Each ROI is 7 voxels, nearly spherical and occupies 189 mm3.
Pre-processing of FC analysis
FC was computed based on multiple regression analyses. Signals were extracted by averaging the time courses from each ROI. To reduce the effects of physiological signals53,54, nine covariates and six motion parameters were added to the regression analysis55,56.
FC analysis of subregions and insular functional integration
Subregion FC analysis included two steps. The FC was first computed between the ROIs. The FC was then calculated between the average signal of the anterior ROIs and the average signal of the posterior ROIs. This latter step of FC analysis was used to investigate the functional integration between the anterior and posterior subregions (hereafter, this FC is simply referred to as insular functional integration).
FC analysis of functional networks
For each ROI, an individual FC network was computed on a voxel-wise basis and corrected according to the false discovery rate (FDR) with p <0.05 and k> 20 voxels. To test the spatial consistency of the FC networks, the probability network was calculated in two steps. The significant voxels were first summed up and the sum was then divided by the number of subjects. This analysis provides a type of population-based spatial consistency. For example, a 40% value in the amateur group indicated that the relevant brain region was activated in 12 subjects [40% = (12/30) × 100%]. Therefore, higher spatial consistency is thought to reflect increased robustness.
GMV analysis of subregions
We used FreeSurfer software to conduct a GMV analysis of the subregions (version 5.0.0; http://surfer.nmr.mgh.harvard.edu). Previous work has validated FreeSurfer by comparing it to histological analysis57 and manual measurements58. The computational steps have been described in detail elsewhere59.
The automated procedure involves segmentation of the WM60, tessellation of the grey matter/white matter (GM/WM) junction, inflation of the folded surface tessellation patterns61 and automatic correction of any topological defects in the resulting manifold. This surface is then utilised as the starting point for a deformable surface algorithm designed to find the GM/WM and pial (GM/cerebrospinal fluid) surfaces with sub-millimetre precision (Fischl and Dale, 2000). For each subject, the cortical surface area and cortical thickness of the cortical ribbon were computed on a uniform grid (comprised of vertices). The cortical thickness is defined as the shortest distance between the GM/WM and pial surface models. The thickness maps produced are not limited to the voxel resolution of the image and are therefore sensitive to sub-millimetre differences between any two groups being compared62. Thickness, surface area and volume measures were mapped onto the inflated surface of each subject's brain reconstruction, allowing visualisation of the data across the entire cortical surface (i.e., gyri and sulci) without being obscured by cortical folding. The reconstructed brain image for each subject was then morphed into an average spherical surface representation that optimally aligned the sulcal and gyral features across subjects61. This procedure accurately matches the morphologically homologous cortical locations among subjects based on their individual anatomy. Finally, maps of the thickness, surface area and volume were created. One of the implemented parcellation schemes in FreeSurfer (aparc.a2009s) was used to compute the five subregions of the insula (short insular gyri, anterior segment of the circular sulcus, long insular gyrus and central sulcus, superior segment of the circular sulcus and inferior segment of the circular sulcus). The entire cortex of each participant was visually inspected and segmentation inaccuracies were manually corrected.
Correlation analysis and comparison between groups
To investigate whether the brain enhancement observed in experts is correlated with AVG playing, we computed pairwise Pearson correlations that included insular functional integration, GMV and the AT. An independent sample t-test was performed between groups. Multiple comparisons were corrected according to the FDR with p <0.05 and a cluster threshold for the FC maps of k> 20.
References
Powers, K. L., Brooks, P. J., Aldrich, N. J., Palladino, M. A. & Alfieri, L. Effects of video-game play on information processing: A meta-analytic investigation. Psychon. Bull. Rev. 20, 1055–1079 (2013).
Green, C. S. & Bavelier, D. Action video game modifies visual selective attention. Nature 423, 534–537 (2003).
Green, C. S. & Bavelier, D. Effect of action video games on the spatial distribution of visuospatial attention. J. Exp. Psychol. Hum. Percept. Perform. 32, 1465–1478 (2006).
Chisholm, J. D., Hickey, C., Theeuwes, J. & Kingstone, A. Reduced attentional capture in action video game players. Atten. Percept. Psychophys. 72, 667–671 (2010).
Anguera, J. et al. Video game training enhances cognitive control in older adults. Nature 501, 97–101, 10.1038/nature12486 (2013).
Fagundo, A. B. et al. Physiological and Brain Activity After a Combined Cognitive Behavioral Treatment Plus Video Game Therapy for Emotional Regulation in Bulimia Nervosa: A Case Report. J. Med. Internet Res. 16, el83 (2014).
Green, C. S. & Bavelier, D. Action-video-game experience alters the spatial resolution of vision. Psychol. Sci. 18, 88–94 (2007).
Donohue, S. E., Woldorff, M. G. & Mitroff, S. R. Video game players show more precise multisensory temporal processing abilities. Atten. Percept. Psychophys. 72, 1120–1129 (2010).
Jones, E., Burton, H., Saper, C. & Swanson, L. Midbrain, diencephalic and cortical relationships of the basal nucleus of Meynert and associated structures in primates. J. Comp. Neurol. 167, 385–419 (1976).
Li, R., Polat, U., Makous, W. & Bavelier, D. Enhancing the contrast sensitivity function through action video game training. Nat. Neurosci. 12, 549–551 (2009).
West, G. L., Al-Aidroos, N. & Pratt, J. Action video game experience affects oculomotor performance. Acta Psychol. (Amst.) 142, 38–42 (2013).
Kennedy, A. M., Boyle, E. M., Traynor, O., Walsh, T. & Hill, A. D. K. Video Gaming Enhances Psychomotor Skills But Not Visuospatial and Perceptual Abilities in Surgical Trainees. J. Surg. Educ. 68, 414–420 (2011).
Granek, J. A., Gorbet, D. J. & Sergio, L. E. Extensive video-game experience alters cortical networks for complex visuomotor transformations. Cortex 46, 1165–1177 (2010).
Mishra, J., Zinni, M., Bavelier, D. & Hillyard, S. A. Neural basis of superior performance of action videogame players in an attention-demanding task. J. Neurosci. 31, 992–998 (2011).
Wu, S. et al. Playing a first-person shooter video game induces neuroplastic change. J. Cognit. Neurosci. 24, 1286–1293 (2012).
Erickson, K. I. et al. Striatal volume predicts level of video game skill acquisition. Cereb. Cortex 20, 2522–2530 (2010).
Kühn, S. et al. The neural basis of video gaming. Transl. Psychiatry 1, e53 (2011).
Tanaka, S. et al. Larger Right Posterior Parietal Volume in Action Video Game Experts: A Behavioral and Voxel-Based Morphometry (VBM) Study. PLoS One 8, e66998 (2013).
Kühn, S. & Gallinat, J. Amount of lifetime video gaming is positively associated with entorhinal, hippocampal and occipital volume. Mol. Psychiatry 19, 842–847 (2013).
Kühn, S., Gleich, T., Lorenz, R., Lindenberger, U. & Gallinat, J. Playing Super Mario induces structural brain plasticity: gray matter changes resulting from training with a commercial video game. Mol. Psychiatry 19, 265–271 (2013).
Nieuwenhuys, R. The insular cortex: a review. Prog. Brain Res. 195, 123–163 (2012).
Cauda, F. et al. Functional connectivity of the insula in the resting brain. NeuroImage 55, 8–23 (2011).
Cerliani, L. et al. Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Hum. Brain Mapp. 33, 2005–2034 (2012).
Cauda, F. et al. Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. NeuroImage 62, 343–355 (2012).
Augustine, J. R. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Rev. 22, 229–244 (1996).
Supekar, K., Musen, M. & Menon, V. Development of large-scale functional brain networks in children. PLoS Biol. 7, e1000157 (2009).
Lewis, C. M., Baldassarre, A., Committeri, G., Romani, G. L. & Corbetta, M. Learning sculpts the spontaneous activity of the resting human brain. Proc. Natl. Acad. Sci. U. S. A. 106, 17558–17563 (2009).
Draganski, B. & May, A. Training-induced structural changes in the adult human brain. Behav. Brain Res. 192, 137–142 (2008).
Boyke, J., Driemeyer, J., Gaser, C., Büchel, C. & May, A. Training-induced brain structure changes in the elderly. J. Neurosci. 28, 7031–7035 (2008).
Corbetta, M. & Shulman, G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 (2002).
Draganski, B. et al. Temporal and spatial dynamics of brain structure changes during extensive learning. J. Neurosci. 26, 6314–6317 (2006).
Giuliani, N. R., Drabant, E. M., Bhatnagar, R. & Gross, J. J. Emotion regulation and brain plasticity: expressive suppression use predicts anterior insula volume. NeuroImage 58, 10–15 (2011).
Veit, R. et al. Using real-time fMRI to learn voluntary regulation of the anterior insula in the presence of threat-related stimuli. Soc. Cogn. Affect. Neurosci. 7, 623–634 (2012).
Hallett, M. Plasticity of the human motor cortex and recovery from stroke. Brain Res. Rev. 36, 169–174 (2001).
Iacoboni, M. & Dapretto, M. The mirror neuron system and the consequences of its dysfunction. Nat. Rev. Neurosci. 7, 942–951 (2006).
Koelsch, S. Toward a neural basis of music perception – a review and updated model. Front. Psychol. 2, 110 (2011).
Allen, J. S., Emmorey, K., Bruss, J. & Damasio, H. Morphology of the insula in relation to hearing status and sign language experience. J. Neurosci. 28, 11900–11905 (2008).
Flynn, F., Benson, D. & Ardila, A. Anatomy of the insula functional and clinical correlates. Aphasiology 13, 55–78 (1999).
Chang, L. J., Yarkoni, T., Khaw, M. W. & Sanfey, A. G. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb. Cortex 23, 739–749 (2013).
Deen, B., Pitskel, N. B. & Pelphrey, K. A. Three systems of insular functional connectivity identified with cluster analysis. Cereb. Cortex 21, 1498–1506 (2011).
Carter, R. M., O'Doherty, J. P., Seymour, B., Koch, C. & Dolan, R. J. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. NeuroImage 29, 1007–1012 (2006).
Hopfinger, J., Buonocore, M. & Mangun, G. The neural mechanisms of top-down attentional control. Nat. Neurosci. 3, 284–291 (2000).
Leung, H. C., Gore, J. & Goldman-Rakic, P. S. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J. Cognit. Neurosci. 14, 659–671 (2002).
Rajah, M. N., Languay, R. & Grady, C. L. Age-related changes in right middle frontal gyrus volume correlate with altered episodic retrieval activity. J. Neurosci. 31, 17941–17954 (2011).
Dosenbach, N. U., Fair, D. A., Cohen, A. L., Schlaggar, B. L. & Petersen, S. E. A dual-networks architecture of top-down control. Trends. Cogn. Sci. 12, 99–105 (2008).
Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 52, 1059–1069 (2010).
Bullmore, E. & Sporns, O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198 (2009).
Corbetta, M. & Shulman, G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 (2002).
Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Elo, A. E. The rating of chessplayers, past and present. Vol. 3 (Arco Pub, 1978).
Du, W. et al. Functional magnetic resonance imaging of brain of college students with internet addiction. Journal of Central South University. Medical sciences 36, 744–749 (2011).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17, 825–841 (2002).
Bandettini, P. A. & Bullmore, E. Endogenous oscillations and networks in functional magnetic resonance imaging. Hum. Brain Mapp. 29, 737 (2008).
Napadow, V. et al. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. NeuroImage 42, 169–177 (2008).
Fox, M. D., Zhang, D., Snyder, A. Z. & Raichle, M. E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 101, 3270–3283 (2009).
Weissenbacher, A. et al. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. NeuroImage 47, 1408–1416 (2009).
Rosas, H. et al. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 58, 695–701 (2002).
Kuperberg, G. R. et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch. Gen. Psychiatry 60, 878 (2003).
Meyer, M., Liem, F., Hirsiger, S., Jancke, L. & Hanggi, J. Cortical Surface Area and Cortical Thickness Demonstrate Differential Structural Asymmetry in Auditory-Related Areas of the Human Cortex. Cereb. Cortex 23, 1–12 (2013).
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage 9, 179–194 (1999).
Fischl, B., Sereno, M. I. & Dale, A. M. Cortical surface-based analysis: II: Inflation, flattening and a surface-based coordinate system. NeuroImage 9, 195–207 (1999).
Fischl. & Dale, A. M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 97, 11050–11055 (2000).
Acknowledgements
We thank Kühn, S. for her invaluable help and advice. This work was supported by the 973 project 2011CB707803, NSFC 81330032, NSFC 91232725.
Author information
Authors and Affiliations
Contributions
D.G. and D.Y. conceived and designed this study. D.G. and H.H. performed the study. H.H., D.L. analyzed the data. D.G., W.M., L.D., C.L. and D.Y. wrote the main manuscript text. D.G. and W.M. prepared the figures and tables. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Gong, D., He, H., Liu, D. et al. Enhanced functional connectivity and increased gray matter volume of insula related to action video game playing. Sci Rep 5, 9763 (2015). https://doi.org/10.1038/srep09763
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep09763
This article is cited by
-
Global visual attention SPAN in different video game genres
Scientific Reports (2023)
-
Long-lasting connectivity changes induced by intensive first-person shooter gaming
Brain Imaging and Behavior (2021)
-
Commercial video games and cognitive functions: video game genres and modulating factors of cognitive enhancement
Behavioral and Brain Functions (2020)
-
Bacomics: a comprehensive cross area originating in the studies of various brain–apparatus conversations
Cognitive Neurodynamics (2020)
-
Narrative and active video game in separate and additive effects of physical activity and cognitive function among young adults
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.