Abstract
Fungi produce millions of clonal asexual conidia (spores) that remain dormant until favourable conditions occur. Conidia contain abundant stable messenger RNAs but the mechanisms underlying the production of these transcripts and their composition and functions are unknown. Here, we report that the conidia of three filamentous fungal species (Aspergillus nidulans, Aspergillus fumigatus, Talaromyces marneffei) are transcriptionally active and can synthesize mRNAs. We find that transcription in fully developed conidia is modulated in response to changes in the environment until conidia leave the developmental structure. Environment-specific transcriptional responses can alter conidial content (mRNAs, proteins and secondary metabolites) and change gene expression when dormancy is broken. Conidial transcription affects the fitness and capabilities of fungal cells after germination, including stress and antifungal drug (azole) resistance, mycotoxin and secondary metabolite production and virulence. The transcriptional variation that we characterize in fungal conidia explains how genetically identical conidia mature into phenotypically variable conidia. We find that fungal conidia prepare for the future by synthesizing and storing transcripts according to environmental conditions present before dormancy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Next-generation sequencing data are available from the National Center for Biotechnology Information Sequence Read Archive database under accession no. PRJNA602418 for the RNAP II ChIP–seq data, PRJNA602550 for the TBP and TFIIB ChIP–seq data, PRJNA602580 for the RNAP I and RNAP III ChIP–seq data, PRJNA602549 for the histone modifications ChIP–seq data and PRJNA607649 for the RNA-seq data. A list of figures that have associated raw data is given in Supplementary Table 10. Gene annotation and GO information were obtained from AspGD (http://www.aspgd.org) and FungiDB (https://fungidb.org). Source data are provided with this paper.
Code availability
All scripts reported in the Methods are available at Github (https://github.com/zqmiao-mzq/closest_gene_calling/blob/master/zqWinSGR-v4.pl; https://github.com/zqmiao-mzq/closest_gene_calling/blob/master/closest_gene_calling_v10.pl; and https://github.com/sethiyap/FungalSporeAnalysis) or at the bioinformatics analysis platform FungiExpresZ (https://cparsania.shinyapps.io/FungiExpresZ/) (Chirag Parsania, unpublished).
References
Lamarre, C. et al. Transcriptomic analysis of the exit from dormancy of Aspergillus fumigatus conidia. BMC Genom. 9, 417 (2008).
Osherov, N. & May, G. S. The molecular mechanisms of conidial germination. FEMS Microbiol. Lett. 199, 153–160 (2001).
Beuchat, L. R. Thermal tolerance of Talaromyces flavus ascospores as affected by growth medium and temperature, age and sugar content in the inactivation medium. Trans. Brit. Mycol. Soc. 90, 359–364 (1988).
Blango, M. G. et al. Dynamic surface proteomes of allergenic fungal conidia. J. Proteome Res. 19, 2092–2104 (2020).
Blaszyk, M., Blank, G., Holley, R. & Chong, J. Reduced water activity during sporogenesis in selected penicillia: impact on spore quality. Food Res. Int. 31, 503–509 (1998).
Bleichrodt, R.-J., Foster, P., Howell, G., Latgé, J.-P. & Read, N. D. Cell wall composition heterogeneity between single cells in Aspergillus fumigatus leads to heterogeneous behavior during antifungal treatment and phagocytosis. mBio 11, e03015-19 (2020).
Cliquet, S. & Jackson, M. Influence of culture conditions on production and freeze-drying tolerance of Paecilomyces fumosoroseus blastospores. J. Ind. Microbiol. Biotechnol. 23, 97–102 (1999).
Cliquet, S. & Jackson, M. A. Impact of carbon and nitrogen nutrition on the quality, yield and composition of blastospores of the bioinsecticidal fungus Paecilomyces fumosoroseus. J. Ind. Microbiol. Biotechnol. 32, 204–210 (2005).
Conner, D. E. & Beuchat, L. R. Efficacy of media for promoting ascospore formation by Neosartorya fischeri, and the influence of age and culture temperature on heat resistance of ascospores. Food Microbiol. 4, 229–238 (1987).
Conner, D. E. & Beuchat, L. R. Heat resistance of ascospores of Neosartorya fischeri as affected by sporulation and heating medium. Int. J. Food Microbiol. 4, 303–312 (1987).
Dagnas, S., Gougouli, M., Onno, B., Koutsoumanis, K. P. & Membre, J.-M. Quantifying the effect of water activity and storage temperature on single spore lag times of three moulds isolated from spoiled bakery products. Int. J. Food Microbiol. 240, 75–84 (2017).
Darby, R. T. & Mandels, G. R. Effects of sporulation medium and age on fungus spore physiology. Plant Physiol. 30, 360–366 (1955).
Hagiwara, D. et al. Temperature during conidiation affects stress tolerance, pigmentation, and trypacidin accumulation in the conidia of the airborne pathogen Aspergillus fumigatus. PLoS ONE 12, e0177050 (2017).
Hallsworth, J. E. & Magan, N. Culture age, temperature, and pH affect the polyol and trehalose contents of fungal propagules. Appl. Environ. Microbiol. 62, 2435–2442 (1996).
Jackson, M. A. & Schisler, D. A. The composition and attributes of Colletotrichum truncatum spores are altered by the nutritional environment. Appl. Environ. Microbiol. 58, 2260–2265 (1992).
Kang, S. E., Celia, B., Bensasson, D. & Monamy, M. Sporulation environment drives phenotypic variation in the pathogen Aspergillus fumigatus. G3 Genes Genom. Genet. https://doi.org/10.1093/g3journal/jkab208 (2021).
Nguyen Van Long, N. et al. Temperature, water activity and pH during conidia production affect the physiological state and germination time of Penicillium species. Int. J. Food Microbiol. 241, 151–160 (2017).
Oliveira, A. S., Braga, G. U. L. & Rangel, D. E. N. Metarhizium robertsii illuminated during mycelial growth produces conidia with increased germination speed and virulence. Fungal Biol. 122, 555–562 (2018).
Oliveira, A. S. & Rangel, D. E. N. Transient anoxia during Metarhizium robertsii growth increases conidial virulence to Tenebrio molitor. J. Invertebr. Pathol. 153, 130–133 (2018).
Oliveira, M., Pereira, C., Bessa, C., Araujo, R. & Saraiva, L. Chronological aging in conidia of pathogenic Aspergillus: comparison between species. J. Microbiol. Methods 118, 57–63 (2015).
Teertstra, W. R. et al. Maturation of conidia on conidiophores of Aspergillus niger. Fungal Genet. Biol. 98, 61–70 (2017).
Takahashi-Nakaguchi, A. et al. Aspergillus fumigatus adhesion factors in dormant conidia revealed through comparative phenotypic and transcriptomic analyses. Cell Microbiol. 20, e12802 (2018).
Wösten, H. A., van Veluw, G. J., de Bekker, C. & Krijgsheld, P. Heterogeneity in the mycelium: implications for the use of fungi as cell factories. Biotechnol. Lett. 35, 1155–1164 (2013).
Ackermann, M. A functional perspective on phenotypic heterogeneity in microorganisms. Nat. Rev. Microbiol. 13, 497–508 (2015).
Avery, S. V. Microbial cell individuality and the underlying sources of heterogeneity. Nat. Rev. Microbiol. 4, 577–587 (2006).
Hewitt, S. K., Foster, D. S., Dyer, P. S. & Avery, S. V. Phenotypic heterogeneity in fungi: importance and methodology. Fungal Biol. Rev. 30, 176–184 (2016).
Kasuga, T. et al. Long-oligomer microarray profiling in Neurospora crassa reveals the transcriptional program underlying biochemical and physiological events of conidial germination. Nucleic Acids Res. 33, 6469–6485 (2005).
van Leeuwen, M. R. et al. Germination of conidia of Aspergillus niger is accompanied by major changes in RNA profiles. Stud. Mycol. 74, 59–70 (2013).
Novodvorska, M. et al. Trancriptional landscape of Aspergillus niger at breaking of conidial dormancy revealed by RNA-sequencing. BMC Genom. 14, 246 (2013).
Zahiri, A. R., Babu, M. R. & Saville, B. J. Differential gene expression during teliospore germination in Ustilago maydis. Mol. Genet. Genomics 273, 394–403 (2005).
Baltussen, T. J. H., Coolen, J. P. M., Zoll, J., Verweij, P. E. & Melchers, W. J. G. Gene co-expression analysis identifies gene clusters associated with isotropic and polarized growth in Aspergillus fumigatus conidia. Fungal Genet. Biol. 116, 62–72 (2018).
Liu, T. et al. The use of global transcriptional analysis to reveal the biological and cellular events involved in distinct development phases of Trichophyton rubrum conidial germination. BMC Genom. 8, 100 (2007).
Inglis, D. O., Voorhies, M., Hocking Murray, D. R. & Sil, A. Comparative transcriptomics of infectious spores from the fungal pathogen Histoplasma capsulatum reveals a core set of transcripts that specify infectious and pathogenic states. Eukaryot. Cell 12, 828–852 (2013).
Hagiwara, D. et al. Comparative transcriptome analysis revealing dormant conidia and germination associated genes in Aspergillus species: an essential role for AtfA in conidial dormancy. BMC Genom. 17, 358 (2016).
Taubitz, A., Bauer, B., Heesemann, J. & Ebel, F. Role of respiration in the germination process of the pathogenic mold Aspergillus fumigatus. Curr. Microbiol. 54, 354–360 (2007).
Novodvorska, M. et al. Metabolic activity in dormant conidia of Aspergillus niger and developmental changes during conidial outgrowth. Fungal Genet. Biol. 94, 23–31 (2016).
Osherov, N., Mathew, J., Romans, A. & May, G. S. Identification of conidial-enriched transcripts in Aspergillus nidulans using suppression subtractive hybridization. Fungal Genet. Biol. 37, 197–204 (2002).
Lara-Rojas, F., Sánchez, O., Kawasaki, L. & Aguirre, J. Aspergillus nidulans transcription factor AtfA interacts with the MAPK SakA to regulate general stress responses, development and spore functions. Mol. Microbiol. 80, 436–454 (2011).
Krijgsheld, P. et al. Development in Aspergillus. Stud. Mycol. 74, 1–29 (2013).
Etxebeste, O., Garzia, A., Espeso, E. A. & Ugalde, U. Aspergillus nidulans asexual development: making the most of cellular modules. Trends Microbiol. 18, 569–576 (2010).
Momany, M., Zhao, J., Lindsey, R. & Westfall, P. J. Characterization of the Aspergillus nidulans septin (asp) gene family. Genetics 157, 969–977 (2001).
Oakley, B. R. Tubulins in Aspergillus nidulans. Fungal Genet. Biol. 41, 420–427 (2004).
Petrenko, N., Jin, Y., Dong, L., Wong, K. H. & Struhl, K. Requirements for RNA polymerase II preinitiation complex formation in vivo. eLife 8, e43654 (2019).
Moreno, M. A. et al. The regulation of zinc homeostasis by the ZafA transcriptional activator is essential for Aspergillus fumigatus virulence. Mol. Microbiol. 64, 1182–1197 (2007).
Champe, S. P., Rao, P. & Chang, A. An endogenous inducer of sexual development in Aspergillus nidulans. J. Gen. Microbiol. 133, 1383–1387 (1987).
Teutschbein, J. et al. Proteome profiling and functional classification of intracellular proteins from conidia of the human-pathogenic mold Aspergillus fumigatus. J. Proteome Res. 9, 3427–3442 (2010).
Wu, M.-Y. et al. Systematic dissection of the evolutionarily conserved WetA developmental regulator across a genus of filamentous fungi. mBio 9, e01130-18 (2018).
Anjo, S. I., Figueiredo, F., Fernandes, R., Manadas, B. & Oliveira, M. A proteomic and ultrastructural characterization of Aspergillus fumigatus’ conidia adaptation at different culture ages. J. Proteomics 161, 47–56 (2017).
Doyle, M. P. & Marth, E. H. Thermal inactivation of conidia from Aspergillus flavus and Aspergillus parasiticus. I. Effects of moist heat, age of conidia, and sporulation medium. J. Milk Food Technol. 38, 678–682 (1975).
Low, S. Y., Dannemiller, K., Yao, M., Yamamoto, N. & Peccia, J. The allergenicity of Aspergillus fumigatus conidia is influenced by growth temperature. Fungal Biol. 115, 625–632 (2011).
Arias, M. et al. Preparations for invasion: modulation of host lung immunity during pulmonary Aspergillosis by gliotoxin and other fungal secondary metabolites. Front. Immunol. 9, 2549 (2018).
Bok, J. W. et al. GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect. Immun. 74, 6761–6768 (2006).
Houšť, J., Spížek, J. & Havlíček, V. Antifungal drugs. Metabolites. 10, 106 (2020).
Bignell, E., Cairns, T. C., Throckmorton, K., Nierman, W. C. & Keller, N. P. Secondary metabolite arsenal of an opportunistic pathogenic fungus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20160023 (2016).
Fernandes, M., Keller, N. P. & Adams, T. H. Sequence-specific binding by Aspergillus nidulans AflR, a C6 zinc cluster protein regulating mycotoxin biosynthesis. Mol. Microbiol. 28, 1355–1365 (1998).
Zadra, I., Abt, B., Parson, W. & Haas, H. xylP promoter-based expression system and its use for antisense downregulation of the Penicillium chrysogenum nitrogen regulator NRE. Appl. Environ. Microbiol. 66, 4810–4816 (2000).
Shaaban, M. I., Bok, J. W., Lauer, C. & Keller, N. P. Suppressor mutagenesis identifies a velvet complex remediator of Aspergillus nidulans secondary metabolism. Eukaryot. Cell 9, 1816–1824 (2010).
Baltussen, T. J. H., Zoll, J., Verweij, P. E. & Melchers, W. J. G. Molecular mechanisms of conidial germination in Aspergillus spp. Microbiol. Mol. Biol. Rev. 84, e00049-19 (2019).
Money, N. P. in The Fungi (eds Watkinson, S. C. et al.) 67–97 (Academic Press, 2016).
Qin, L. et al. Universal plasmids to facilitate gene deletion and gene tagging in filamentous fungi. Fungal Genet. Biol. 125, 28–35 (2019).
Todd, R. B., Davis, M. A. & Hynes, M. J. Genetic manipulation of Aspergillus nidulans: heterokaryons and diploids for dominance, complementation and haploidization analyses. Nat. Protoc. 2, 822–830 (2007).
Fan, X., Lamarre-Vincent, N., Wang, Q. & Struhl, K. Extensive chromatin fragmentation improves enrichment of protein binding sites in chromatin immunoprecipitation experiments. Nucleic Acids Res. 36, e125 (2008).
Wong, K. H. & Struhl, K. The Cyc8–Tup1 complex inhibits transcription primarily by masking the activation domain of the recruiting protein. Genes Dev. 25, 2525–2539 (2011).
Wong, K. H., Jin, Y. & Moqtaderi, Z. Multiplex Illumina sequencing using DNA barcoding. Curr. Protoc. Mol. Biol. 101, 7.11.1–7.11.11 (2013).
Rio, D. C., Ares, M.Jr., Hannon, G. J. & Nilsen, T. W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 6, pdb-prot5439 (2010).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Zhang, Y. et al. Model-based analysis of ChIP–Seq (MACS). Genome Biol. 9, R137 (2008).
Pertea, M., Kim, D., Pertea, G. M., Leek, J. T. & Salzberg, S. L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 11, 1650–1667 (2016).
Ashburner, M. et al. Gene Ontology: tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
Stajich, J. E. et al. FungiDB: an integrated functional genomics database for fungi. Nucleic Acids Res. 40, D675–D681 (2012).
Rueden, C. T. et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 18, 529 (2017).
Maleszka, R. & Pieniazek, N. J. Modified replica plating technique of microcolonies of Aspergillus nidulans using Triton-X100. Asp. Newsl. 15, 36–38 (1981).
Rakotonirainy, M. S., Héraud, C. & Lavédrine, B. Detection of viable fungal spores contaminant on documents and rapid control of the effectiveness of an ethylene oxide disinfection using ATP assay. Luminescence 18, 113–121 (2003).
Wong, K. H., Hynes, M. J., Todd, R. B. & Davis, M. A. Transcriptional control of nmrA by the bZIP transcription factor MeaB reveals a new level of nitrogen regulation in Aspergillus nidulans. Mol. Microbiol. 66, 534–551 (2007).
Stunnenberg, H. G., Wennekes, L. M., Spierings, T. & van den Broek, H. W. An alpha-amanitin-resistant DNA-dependent RNA polymerase II from the fungus Aspergillus nidulans. Eur. J. Biochem. 117, 121–129 (1981).
Bensaude, O. Inhibiting eukaryotic transcription: which compound to choose? How to evaluate its activity? Transcription 2, 103–108 (2011).
Acknowledgements
We thank members of the Wong laboratory for comments and discussions throughout the study and M. J. Hynes, A. Andrianopoulos, R. B. Todd, M. Momany and K. Struhl for insightful discussions. We acknowledge the services and technical supports from the Genomics and Single Cell Analysis Core and the Drug and Development Core of the Faculty of Health Sciences at the University of Macau. This work was performed in part at the High-Performance Computing Cluster (HPC), which is supported by the Information and Communication Technology Office (ICTO) of the University of Macau. We thank L. Pardeshi and N. Shirgaonkar from the Genomics, Bioinformatics and Single Cell Analysis Core and Z. Miao and C. Parsania for Bioinformatics support, and J. Chan from ICTO for technical support on the HPC. We also acknowledge the support from the Science and Technology Development Fund, Macao S.A.R (FDCT) (project no. 0106/2020/A), the Research Services and Knowledge Transfer Office (project nos. MYRG2018-00017-FHS and MYRG2019-00099-FHS) and Faculty of Health Sciences of the University of Macau to K.H.W.
Author information
Authors and Affiliations
Contributions
F.W. and K.H.W. conceived the study, designed the experiments and interpreted the data. F.W. performed the experiments. F.W. performed the bioinformatics analysis with help from P.S. K.T. performed the Illumina sequencing and provided technical support. F.W. and X.H. performed the LC–MS analysis. F.W., S.G., Y.C. and A.L. engineered the strains. K.H.W. provided the funding. F.W. and K.H.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Microbiology thanks Jan Dijksterhuis, Jean-Paul Latge and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Conidia on conidiophore have robust transcription activities.
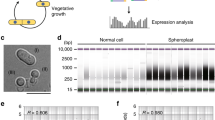
a, Genome browser screenshots showing RNAP II ChIPseq and input DNA signals at selected genes with differential RNAP II occupancies for conidia and hyphae of A. nidulans, A. fumigatus and T. marneffei. b, Heatmap plots of RNAP II ChIPseq and input DNA signals at all annotated genes for conidia of the three species. The number of RNAP II occupied genes for each species is indicated on the colour lines at the right. c, A schematic diagram showing statistically significant (p-value < 0.01) GO terms enriched among transcribed genes for the three species. Colour intensity and size of diamonds indicate p-value and percentage of background, respectively. The p-value was calculated by Fisher’s Exact test and was corrected for multiple testing using Benjamini-Hochberg false discovery rate and the Bonferroni method. d-f, Heatmap plots showing RNAP II ChIPseq signal at (d) conidia-enriched-transcripts (CET), conidial maturation and trehalose biosynthesis genes, (e) conidiation initiation genes, and (f) septin and β-tubulin genes for A. nidulans conidia and hyphae. Input DNA signals for conidia were included as a negative control. g, A representative microscopy image example for a conidial preparation of A. nidulans for ChIPseq and other experiments. The experiment was repeated three independent times with similar results, and representative image was presented. h-j, Boxplots showing (h) RNAP III (TFIIIBHA and Rpo31HA), (i) RNAP I (Rpc40HA and Rpa43HA) and (j) RNAP II binding signals at (h) tRNAs (n = 180) and 5 S rDNAs (n = 52), (i) rDNAs (n = 16) and (j) protein encoding genes (n = 1,030) in A. nidulans conidia and hyphae. Lower and upper hinges of boxes represent 25th and 75th percentiles, respectively, and the centre of boxes represents the median. The p-values shown were calculated using two samples t-test by ggplot2. The asterisks *, **, *** and **** depict p-values of <0.05, <0.005, <0.001 and <0.0001, respectively, while ns represents a p-value of >0.05. k, A scatter plot showing the comparable mRNA levels of ribosomal protein genes detected by RNAseq in conidia and hyphae.
Extended Data Fig. 2 A. nidulans conidia transcriptionally respond to the environment, activating condition-specific physiological pathways.
a-b, Heatbox plots showing (a) RNAP II binding and (b) mRNA levels of heat-shock response genes in conidia subjected to heat (42 °C) for 0.5 or 4 h as measured by (a) ChIP-qPCR and (b) RT-PCR, respectively. c, A heatbox plot showing RNAP II binding level as measured by ChIP-qPCR of heat shock response genes in hyphae after heat-shock treatment (4 h). d, Schematic diagrams showing GO analysis of differentially transcribed genes for A. nidulans conidia subjected to temperature shocks (4 and 42 °C) and control conidia (37 °C) or between A. nidulans conidia grown in the presence of sodium chloride (NaCl) or potassium chloride (KCl) or in the absence of zinc (Zinc-starved) comparing to control conidia from ANM media. Colour intensity and size of diamonds represent p-value (expressed as –log10p-value) and percentage of background. The p-value was calculated by Fisher’s Exact test and was corrected for multiple testing using Benjamini-Hochberg false discovery rate and the Bonferroni method. e, Heatbox plots showing the RNAP II binding level of glycerol transport and oxidative stress response genes in NaCl and KCl conidia compared to Control conidia.
Extended Data Fig. 3 A. fumigatus conidia transcriptionally respond to the environment, activating condition-specific physiological pathways.
a, A heatmap plot showing differential expressed genes (presented as z-scores) among A. fumigatus conidia formed from ANM media in the presence of sodium chloride (NaCl) and potassium chloride (KCl) or in the absence of zinc (Zinc-starved). b, Schematic diagrams showing gene ontology analysis of differentially transcribed genes for A. fumigatus conidia grown in the presence of sodium chloride (NaCl) or potassium chloride (KCl) or in the absence of zinc (Zinc-starved), as compared to control conidia from ANM media. Colour intensity and size of diamonds represent p-value (expressed as –log10p-value) and percentage of background. The p-value was calculated by Fisher’s Exact test and was corrected for multiple testing using Benjamini-Hochberg false discovery rate and the Bonferroni method.
Extended Data Fig. 4 A. fumigatus conidia have transcription and ATP-consuming activities even after conidial development has completed.
a, Genome browser screenshots showing RNAP II ChIPseq and input DNA signals at selected genes with differential RNAP II occupancies for 3 and 17 day-old conidia. b, Heatmap plots showing RNAP II ChIPseq signals at genes occupied by RNAP II in either 3 or 17 day-old conidia. c, Heatmap plot showing differential expressed genes between 3 or 17 day-old conidia. d-f, Bar plots showing (d) the number of conidia, (e) viability and (f) intracellular ATP levels for conidia of different developmental ages (3, 6 or 17 day-old). d-f Data are presented as mean values + SD. Error bars represent the SD of three independent experiments (n = 3).
Extended Data Fig. 5 Conidial ATP level is linked to conidial viability in A. nidulans.
a-b, Bar graphs showing the mean (a) conidia numbers and (b) intracellular conidial ATP levels after the indicated time (that is, developmental age of conidia; 3, 6, 10, 20, 30, 40, 50, 60 and 70 day after inoculation) from two independent experiments. The same trend was observed in the biological repeats and the raw data are presented in Source Data Extended Data Fig. 5. c, A bar graph showing the viability of conidia same as (a-b). Data are presented as mean values + SD. Error bars represent the standard deviation of three independent experiments (n = 3). d, A gel electrophoresis result showing the quality and yield of chromatin extracted from conidia of different developmental ages collected in parallel with those conidia analyzed for a-c. e, A scatter plot showing the Pearson Correlation analysis of mRNA levels measured by RNAseq in attached and separated conidia as in Fig. 3h.
Extended Data Fig. 6 Overlaps between RNAP II bound genes and targets of AtfA and WetA in A. nidulans conidia.
a, A Venn diagram showing the overlap of genes occupied by RNAP II in conidia and binding targets (by ChIPseq) of transcription factor AtfAMYC and WetAMYC in conidia. b, A scatter plot showing Pearson Correlation of RNAP II occupancies and mRNA levels for AtfA and WetA common ChIPseq binding targets (n = 536) in conidia. c, Heatmap plots showing ChIPseq signals of RNAP II, WetAMYC and AtfAMYC for selected conidium-specific genes in A. nidulans.
Extended Data Fig. 7 A. nidulans and A. fumigatus conidia formed from different conditions have dissimilar transcriptional response and protein expression patterns.
a-d, Heatbox plots showing RNAP II ChIPseq signals for genes encoding (a) conidia surface and intracellular proteins, (b) cell wall proteins, (c) copper and (d) sugar transporters in A. nidulans conidia formed under zinc deficiency (Zinc-starved conidia) or in the presence or absence (Control conidia) of NaCl and KCl (NaCl- and KCl-conidia, respectively). e, Western blot analysis showing the protein expression level of sugar transporters (for example XtrE and MstB) in control and NaCl conidia of A. nidulans. Histone H3 was used as a loading control. The Western blot experiment was repeated three independent times with similar result. f, Heatbox plots showing RNAP II ChIPseq signals for genes encoding conidia surface and intracellular proteins, dehydrin-like proteins, allergens and transporters in A. fumigatus conidia formed under zinc deficiency (Zinc-starved conidia) or in the presence or absence (Control conidia) of NaCl and KCl (NaCl- and KCl-conidia, respectively). Results of A. fumigatus are marked by green-shaded background.
Extended Data Fig. 8 Disparities between transcription, mRNA and protein levels in A. nidulans conidia.
a, Western Blotting results showing protein levels of representative genes in A. nidulans conidia and hyphae. The Western blot experiment was repeated three independent times with similar result. b, Heatbox plots showing RNAP II ChIPseq and mRNA levels for the genes encoding those proteins analysed in (a) in conidia and hyphae.
Extended Data Fig. 9 Sporulation conditions and conidial age affect the transcription levels of transcription factor-encoding genes, protein expression efficiency following germination and germination behaviours of A. nidulans and A. fumigatus conidia.
a-b, Line plots showing the quantification of protein bands in the Western Blotting time-course analysis presented in Fig. 4f and g for (a) Hsp70 protein in heat-shocked and cold-shocked conidia during germination at high temperature (for example 42 °C) and (b) ZapA protein in control and zinc-starved conidia before and during germination in the absence of zinc. Histone H3 was used as a loading control. c, A heatbox plot showing the transcription levels for 48 transcription factor (TF)-encoding genes, which have detectable RNAP II ChIPseq signals, in Zinc-starved, NaCl-, KCl- and Control conidia of A. fumigatus. d-e, Line plots showing the germination kinetics of A. nidulans (d) conidia of different developmental ages (3 and 17 day-old) and (e) conidia formed under ANM media (Control) in the presence of sodium chloride (NaCl) or in the absence of zinc (Zinc-starved). Error bars represent the standard deviation of three independent experiments. The p-values shown were calculated using (d) unpaired t-test in two-tailed or (e) one way ANOVA with multiple comparisons test. f-g, Bar plots showing percentage of germination for A. nidulans conidia formed under the indicated conditions as in (f) and (g) at various germination times (for example 4 h, 4.5 h, 5 h, 6 h and 7 h). d-g, Data are presented as mean values + SD. Error bars represent the SD of three independent experiments (n = 3). The asterisks *, **, *** and **** depict p-values of <0.05, <0.005, <0.001 and <0.0001, respectively, while ns represents a p-value of >0.05. Results of A. fumigatus are marked by green-shaded background.
Extended Data Fig. 10 Conidia formed under different sporulation conditions have different transcription levels for genes of various physiological pathways.
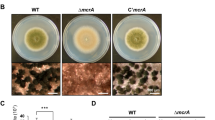
a-b, Heatbox plots showing RNAP II ChIPseq signals and expression changes of (a) oxidative stress response genes and (b) ergosterol biosynthesis genes for A. nidulans conidia formed under ANM media (Control) and ANM media with sodium chloride (NaCl). c-d, Growth tests of A. nidulans wildtype strains on solid ANM media containing different concentrations of the azole-related anti-fungal drugs (c) itraconazole (0, 0.05 and 0.1 µg/ml) and voriconazole (0, 0.05 and 0.1 µg/ml), and the non-azole-related anti-fungal drugs (d) caspofungin (0.2, 0.8 and 2.4 µg/ml) and flucytosine (10, 30 and 90 µg/ml) using conidia formed under ANM media containing sodium chloride (NaCl conidia) or not (Control conidia). e, Heatbox plots showing RNAP II ChIPseq signals and expression changes of gliotoxin protection and control genes for A. fumigatus conidia formed on ANM media with (Control) and without zinc (Zinc-starved). f, Heatbox plots showing expression difference of annotated secondary metabolite biosynthesis clusters genes in A. fumigatus between conidia formed under ANM media with (Control) and without zinc (Zinc-starved). g, Microscopy images showing the conidiation phenotype of the xylP(p)aflR strain with different levels of xylose induction (0, 0.1, 0.2, 0.4, 0.6, 0.8 and 1%). Results of A. fumigatus are marked by green-shaded background. The experiment was performed three independent times with similar results and representative images are presented.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3.
Supplementary Tables 1–12
Supplementary Table 1: Genes with positive elongating RNAP II ChIP–seq signals in conidia and hyphae for Aspergillus nidulans, Aspergillus fumigatus and Talaromyces marneffei. Supplementary Table 2: GO analysis outputs for genes with positive elongating RNAP II ChIP–seq signals in conidia for A. nidulans, A. fumigatus and T. marneffei. Supplementary Table 3: Differentially expressed genes for conidia exposed to temperature shocks (for example, 4 or 42 °C) or conidia formed under different media (for example, ANM media with sodium chloride (NaCl) or potassium chloride (KCl) or without zinc) compared to control conidia (37 °C on ANM media). Supplementary Table 4: GO analysis outputs for genes transcribed in conidia exposed to temperature shocks (for example, 4 or 42 °C) or conidia formed under different media (for example, ANM media with sodium chloride (NaCl) or potassium chloride (KCl) or without zinc (zinc-starved)). Supplementary Table 5: Summary of transcription and mRNA levels for genes presented in Extended Data Fig. 8b. Supplementary Table 6: Strains used in this project. Supplementary Table 7: Summary of conidia treatments used in this study. Supplementary Table 8: Antibodies used in this project. Supplementary Table 9: Primers used in this project. Supplementary Table 10: List of figures that have associated raw sequencing data. Supplementary Table 11: Summary of the number of mapped reads and read length for each next-generation sequencing data sample. Supplementary Table 12: Raw data used in Supplementary Fig. 3a–c.
Source data
Source Data Fig. 3
Unprocessed immunoblots for Fig. 3i,j.
Source Data Fig. 4
Unprocessed immunoblots for Fig. 4b,f,g.
Source Data Extended Data Fig. 5
Unprocessed gel images for Extended Data Fig. 5d.
Source Data Extended Data Fig. 7
Unprocessed immunoblots for Extended Data Fig.7e.
Source Data Extended Data Fig. 8
Unprocessed immunoblots for Extended Data Fig. 8a.
Source Data Fig. 3
Raw data used in Fig. 3c,d,g,h,k.
Source Data Fig. 4
Raw data used in Fig. 4a,d,h,i.
Source Data Fig. 5
Raw data used in Fig. 5h,i,k.
Source Data Extended Data Fig. 4
Raw data used in Extended Data Fig. 4d–f.
Source Data Extended Data Fig. 5
Raw data used in Extended Data Fig. 5a–c.
Source Data Extended Data Fig. 9
Raw data used in Extended Data Fig. 9f,g.
Rights and permissions
About this article
Cite this article
Wang, F., Sethiya, P., Hu, X. et al. Transcription in fungal conidia before dormancy produces phenotypically variable conidia that maximize survival in different environments. Nat Microbiol 6, 1066–1081 (2021). https://doi.org/10.1038/s41564-021-00922-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-021-00922-y
This article is cited by
-
Morphology, Development, and Pigment Production of Talaromyces marneffei are Diversely Modulated Under Physiologically Relevant Growth Conditions
Current Microbiology (2024)
-
Compatible solutes determine the heat resistance of conidia
Fungal Biology and Biotechnology (2023)
-
C-terminus of serine–arginine protein kinase-like protein, SrpkF, is involved in conidiophore formation and hyphal growth under salt stress in Aspergillus aculeatus
International Microbiology (2023)
-
Biopolymer-based emulsions for the stabilization of Trichoderma atrobrunneum conidia for biological control
Applied Microbiology and Biotechnology (2023)
-
Evolution of the human pathogenic lifestyle in fungi
Nature Microbiology (2022)