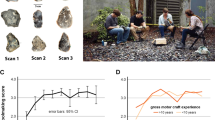
Abstract
After 800,000 years of making simple Oldowan tools, early humans began manufacturing Acheulian handaxes around 1.75 million years ago. This advance is hypothesized to reflect an evolutionary change in hominin cognition and language abilities. We used a neuroarchaeology approach to investigate this hypothesis, recording brain activity using functional near-infrared spectroscopy as modern human participants learned to make Oldowan and Acheulian stone tools in either a verbal or nonverbal training context. Here we show that Acheulian tool production requires the integration of visual, auditory and sensorimotor information in the middle and superior temporal cortex, the guidance of visual working memory representations in the ventral precentral gyrus, and higher-order action planning via the supplementary motor area, activating a brain network that is also involved in modern piano playing. The right analogue to Broca’s area—which has linked tool manufacture and language in prior work1,2—was only engaged during verbal training. Acheulian toolmaking, therefore, may have more evolutionary ties to playing Mozart than quoting Shakespeare.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Stout, D., Toth, N., Schick, K. D. & Chaminade, T. Neural correlates of Early Stone Age tool-making: technology, language and cognition in human evolution. Phil. Trans. R. Soc. B 363, 1939–1949 (2008).
Stout, D. & Chaminade, T. Stone tools, language and the brain in human evolution. Phil. Trans. R. Soc. B 367, 75–87 (2012).
Schoenemann, P. T. Evolution of the size and functional areas of the human brain. Annu. Rev. Anthropol. 35, 379–406 (2006).
Sherwood, C. C., Subiaul, F. & Zawadzki, T. W. A natural history of the human mind: tracing evolutionary changes in brain and cognition. J. Anat. 212, 426–454 (2008).
Stout, D. & Hecht, E. in Human Paleoneurology (ed. Bruner, E.) 145–175 (Springer, 2015).
Binneman, J. & Beaumont, P. Use-wear analysis of two Acheulean handaxes from Wonderwerk Cave, Northern Cape. South Afr. Field Archaeol. 1, 92–97 (1992).
Domínguez-Rodrigo, M., Serrallonga, J., Juan-Tresserras, J., Alcala, L. & Luque, L. Woodworking activities by early humans: a plant residue analysis on Acheulian stone tools from Peninj (Tanzania). J. Hum. Evol. 40, 289–299 (2001).
Solodenko, N. et al. Fat residue and use-wear found on Acheulian biface and scraper associated with butchered elephant remains at the site of Revadim, Israel. PLoS ONE 10, e0118572 (2015).
Balzeau, A., Holloway, R. L. & Grimaud-Hervé, D. Variations and asymmetries in regional brain surface in the genus Homo. J. Hum. Evol. 62, 696–706 (2012).
Wynn, T. The intelligence of Later Acheulean hominids. Man 14, 371–391 (1979).
Stout, D., Hecht, E., Nada, K., Bradley, B. & Chaminade, T. Cognitive demands of Lower Paleolithic toolmaking. PLoS ONE 10, e0121804 (2015).
Putt, S. S. Human Brain Activity during Stone Tool Production: Tracing the Evolution of Cognition and Language. PhD thesis, Iowa Univ. (2016).
Mahaney, R. A. Exploring the complexity and structure of Acheulean stoneknapping in relation to natural language. Paleoanthropol. 2014, 586–606 (2014).
Stout, D. & Chaminade, T. The evolutionary neuroscience of tool making. Neuropsychologia 45, 1091–1100 (2007).
Stout, D., Passingham, R., Frith, C., Apel, J. & Chaminade, T. Technology, expertise and social cognition in human evolution. Eur. J. Neurosci. 33, 1328–1338 (2011).
Morgan, T. J. H. et al. Experimental evidence for the co-evolution of hominin tool-making teaching and language. Nat. Commun. 6, 6029 (2015).
Uomini, N. T. & Meyer, G. F. Shared brain lateralization patterns in language and Acheulean stone tool production: a functional transcranial Doppler ultrasound study. PLoS ONE 8, e72693 (2013).
Putt, S. S., Woods, A. D. & Franciscus, R. G. The role of verbal interaction during experimental bifacial stone tool manufacture. Lithic Technol. 39, 96–112 (2014).
Wijeakumar, S., Spencer, J. P., Bohache, K., Boas, D. A. & Magnotta, V. A. Validating a new methodology for optical probe design and image registration in fNIRS studies. Neuroimage 106, 86–100 (2015).
Wijeakumar, S., Huppert, T., Magnotta, V. A., Buss, A. T. & Spencer, J. P. Validating an image-based fNIRS approach with fMRI and a working memory task. Neuroimage 147, 204–218 (2016).
Wynn, T. & Coolidge, F. L. The implications of the working memory model for the evolution of modern cognition. Int. J. Evol. Biol. 2011, 741357 (2011).
Vouloumanos, A., Kiehl, K. A., Werker, J. F. & Liddle, P. F. Detection of sounds in the auditory stream: event-related fMRI evidence for differential activation to speech and nonspeech. J. Cogn. Neurosci. 13, 994–1005 (2001).
Lewis, W. Cortical networks related to human use of tools. Neuroscientist 12, 211–231 (2006).
Leff, A. P. et al. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain 132, 3401–3410 (2009).
Goldberg, G. Supplementary motor area structure and function: review and hypotheses. Behav. Brain Sci. 8, 567–588 (1985).
Augustine, J. R. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Rev. 22, 229–244 (1996).
Menon, V. & Uddin, L. Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667 (2010).
Stout, D., Apel, J., Commander, J. & Roberts, M. Late Acheulean technology and cognition at Boxgrove, UK. J. Archaeol. Sci. 41, 576–590 (2014).
Gowlett, J. A. J. in Lucy to Language: The Benchmark Papers (eds Dunbar, R. I. M., Gamble, C. & Gowlett, J. A. J.) Ch. 18 (Oxford Univ. Press, 2014).
Bangert, M. et al. Shared networks for auditory and motor processing in professional pianists: evidence from fMRI conjunction. Neuroimage 30, 917–926 (2006).
Wong, C. & Gallate, J. The function of the anterior temporal lobe: a review of the empirical evidence. Brain Res. 1449, 94–116 (2012).
Pascual, B., et al. Large-scale brain networks of the human left temporal pole: a functional connectivity MRI study. Cereb. Cortex 25, 680–702 (2015).
Rogers, R. D. et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J. Neurosci. 19, 9029–9038 (1999).
Vigneau, M. et al. What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing? Insights from a meta-analysis. Neuroimage 54, 577–593 (2011).
Levy, B. J. & Wagner, A. D. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann. NY Acad. Sci. 1224, 40–62 (2011).
Corbetta, M. & Schulman, G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 (2002).
Hauk, O., Johnsrude, I. & Pulvermüller, F. Somatotopic representation of action words in human motor and premotor cortex. Neuron 41, 301–307 (2004).
Carvalho, S., Cunha, E., Sousa, C. & Matsuzawa, T. Chaînes opératoires and resource-exploitation strategies in chimpanzee (Pan troglodytes) nut cracking. J. Hum. Evol. 55, 148–163 (2008).
Proffitt, T. et al. Wild monkeys flake stone tools. Nature 539, 85–88 (2016).
Martínez, I. et al. Communicative capacities in Middle Pleistocene humans from the Sierra de Atapuerca in Spain. Quat. Int. 295, 94–101 (2013).
Quam, R. M. et al. Early hominin auditory ossicles from South Africa. Proc. Natl Acad. Sci. USA 110, 8847–8851 (2013).
Antón, S. C. Potts, R. & Aiello, L. C. Evolution of early Homo: an integrated biological perspective. Science 345, 1236828 (2014).
Arbib, M. A. From mirror neurons to complex imitation in the evolution of language and tool use. Annu. Rev. Anthropol. 40, 257–273 (2011).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences 2nd edn (Laurence Erlbaum Associates, 1988).
Soper, D. S. A-priori sample size calculator for Student’s t-tests. http://www.danielsoper.com/statcalc/calculator.aspx?id=47 (2017).
Szaflarski, J. P. et al. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology 59, 238–244 (2002).
Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Skinner, H. A. The drug abuse screening test. Addict Behav 7, 363–371 (1982)
London, E. D., Ernst, M., Grant, S., Bonson, K. & Weinstein, A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb. Cortex 10, 334–342 (2000).
Yankosec, K. E. & Howell, D. A narrative review of dexterity assessments. J. Hand Ther. 22, 258–270 (2009).
Desrosiers, J., Rochette, A., Hebert, R. & Bravo, G. The Minnesota manual dexterity test: reliability, validity and reference values studies with healthy elderly people. Can. J. Occup. Ther. 64, 270–276 (1997).
Toth, N. The Oldowan reassessed: a close look at early stone artifacts. J. Archaeol. Sci. 12, 101–120 (1985).
Stout, D. Stone toolmaking and the evolution of human culture and cognition. Phil. Trans R. Soc. B 366, 1050–1059 (2011).
Hoard, R. J. & Anglen, A. A. Lithic analysis. Plains Anthropol. 48, 36–50 (2003).
Andrefsky, W. Lithics: Macroscopic Approaches to Analysis 2nd edn (Cambridge Univ. Press, 2005).
Toth, N., Schick, K. & Semaw, S. In The Oldowan: Case Studies into the Earliest Stone Age (Stone Age Institute, 2006).
Bamforth, D. B. & Finlay, N. Introduction: archaeological approaches to lithic production skill and craft learning. J. Archaeol. Method Theory 15, 1–27 (2008).
Badre, D. & Wagner, A. D. Frontal lobe mechanisms that resolve proactive interference. Cereb. Cortex 15, 2003–2012 (2005).
Pessoa, L., Gutierrez, E., Bandettini, P. A. & Ungerleider, L. G. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron 35, 975–987 (2002).
Pessoa, L. & Ungerleider, L. G. Neural correlates of change detection and change blindness in a working memory task. Cereb. Cortex 14, 511–520 (2004).
Huppert, T. J ., Diamond, S. G ., Franceschini, M. A. & Boas, D. A. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Optics 48, D280–D298 (2009).
Yücel, M. A., Selb, J., Cooper, R. J. & Boas, D. A. Targeted principle component analysis: a new motion artifact correction approach for near-infrared spectroscopy. J. Innovat. Opt. Health Sci. 07, 1350066 (2014).
Fang, Q. & Boas, D. Monte Carlo simulation of photon migration in 3D turbid media accelerated by graphics processing units. Opt. Express 17, 20178–20190 (2009).
Tikhonov, A. Solution of incorrectly formulated problems and the regularization method. Soviet Math. Dokl. 5, 1035–1038 (1963).
Calvetti, D., Morigi, S., Reichel, L. & Sgallari, F. Tikhonov regularization and the L-curve for large discrete ill-posed problems. J. Comput. Appl. Math. 123, 423–446 (2000).
Chen, G., Adleman, N. E., Saad, Z. S., Leibenluft, E. & Cox, R. W. Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. Neuroimage 99, 571–588 (2014).
Acknowledgements
We thank A. Woods for his time and knapping expertise, the Stone Age Institute for its contributions to Fig. 1, and D. Jones, C. Daniel, E. DeForest, A. Vega, N. Fox, A. Wells, E. Hoeper, E. Dellopolous, M. Adams, S. Allchin and G. Brua for their assistance in the lab. S.S.P. acknowledges support from the Wenner–Gren Foundation (8968), Leakey Foundation, Sigma Xi, the Scientific Research Society and the University of Iowa. S.S.P. held an American Fellowship from AAUW. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
S.S.P. and R.G.F. developed the concept for the study. S.S.P. and J.P.S. designed the study. S.S.P. performed the experiment and carried out the analyses, under the supervision of S.W. and J.P.S. S.S.P. and J.P.S. wrote the manuscript, with contributions from S.W. and R.G.F.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Supplementary Information
Supplementary Discussion, Supplementary Tables 1-3, Supplementary Figures 1-3, Supplementary References. (PDF 424 kb)
Rights and permissions
About this article
Cite this article
Putt, S., Wijeakumar, S., Franciscus, R. et al. The functional brain networks that underlie Early Stone Age tool manufacture. Nat Hum Behav 1, 0102 (2017). https://doi.org/10.1038/s41562-017-0102
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41562-017-0102
This article is cited by
-
Language evolution and computational capabilities: conceptualization of the first language units
International Journal of Anthropology and Ethnology (2023)
-
From fossils to mind
Communications Biology (2023)
-
Neuroplasticity enables bio-cultural feedback in Paleolithic stone-tool making
Scientific Reports (2023)
-
The role of the anterior temporal cortex in action: evidence from fMRI multivariate searchlight analysis during real object grasping
Scientific Reports (2022)
-
Inferring intelligence of ancient people based on modern genomic studies
Journal of Human Genetics (2022)