Abstract
The tumor necrosis factor (TNF) receptor superfamily member 11a (TNFRSF11a, also known as RANK) was demonstrated to play an important role in tumor metastasis. However, the specific function of RANK in colorectal cancer (CRC) metastasis and the underlying mechanism are unknown. In this study, we found that RANK expression was markedly upregulated in CRC tissues compared with that in matched noncancerous tissues. Increased RANK expression correlated positively with metastasis, higher TNM stage, and worse prognosis in patients with CRC. Overexpression of RANK promoted CRC cell metastasis in vitro and in vivo, while knockdown of RANK decreased cell migration and invasion. Mechanistically, RANK overexpression significantly upregulated the expression of tartrate-resistant acid phosphatase 5 (TRAP/ACP5) in CRC cells. Silencing of ACP5 in RANK-overexpressing CRC cells attenuated RANK-induced migration and invasion, whereas overexpression of ACP5 increased the migration and invasion of RANK-silencing cells. The ACP5 expression was transcriptionally regulated by calcineurin/nuclear factor of activated T cells c1 (NFATC1) axis. The inhibition of calcineurin/NFATC1 significantly decreased ACP5 expression, and attenuated RANK-induced cell migration and invasion. Furthermore, RANK induced phospholipase C-gamma (PLCγ)-mediated inositol-1,4,5-trisphosphate receptor (IP3R) axis and stromal interaction molecule 1 (STIM1) to evoke calcium (Ca2+) oscillation. The RANK-mediated intracellular Ca2+ mobilization stimulated calcineurin to dephosphorylate NFATC1 and induce NFATC1 nuclear translocation. Both blockage of PLCγ-IP3R axis and STIM1 rescued RANK-induced NFATC1 nuclear translocation, ACP5 expression, and cell metastasis. Our study revealed the functional expression of RANK in human CRC cells and demonstrated that RANK induced the Ca2+-calcineurin/NFATC1-ACP5 axis in the regulation of CRC metastasis, that might be amenable to therapeutic targeting.
Similar content being viewed by others
Introduction
The tumor necrosis factor (TNF) receptor superfamily member 11a (TNFRSF11a, also known as RANK) and its ligand TNF superfamily member 11 (also known as RANKL) were first identified as pivotal regulators of osteoclast development in the late 1990s (ref. 1). Membrane-bound or soluble RANKL from osteoblasts interacts with RANK-expressing pre-osteoclasts to induce osteoclast differentiation for bone remodeling2. Over the past two decades the RANKL/RANK axis has been identified as a critical signaling pathway involved in several mechanisms beyond bone homeostasis, most notably in cancer cell migration and bone development3. Abundant RANKL in the bone environment was identified early as a chemoattractant for bone-specific metastasis of epithelial tumors and melanoma that expressed RANK4. Luo et al.5 proposed that RANKL from tumor-infiltrating inflammatory cells activated RANK on prostate cancer and led to nuclear inhibitor of nuclear factor kappa-B (NF-κB) kinase subunit alpha (IKKα) activation to control cancer metastasis. Later their group found that tumor-infiltrating CD4+CD25+FOXP3+ T cells were a major source of RANKL production in breast cancer and stimulated breast cancer metastasis through RANKL–RANK signaling6. Furthermore, RANK is also involved in the pathogenesis of other solid tumors and nonsolid cancers7. Indeed, 89% of carcinomas assessed were RANK immunostaining positive and ~60% of cases exhibited >50% of RANK-positive cancer cells8,9. Studies have proven that increased RANK expression in tumors correlates with worse clinical parameters and progression8. The mechanisms by which RANK participates in the cancer development include bone environment-dependent effects and direct RANK signaling. Based on Stephen Paget’s seed and soil theory10, on the one hand, RANKL expressed by osteoblasts and stromal cells in bone is an important chemoattractant for tumor bone metastasis. On the other hand, tumor-associated factors secreted from cancer cells enhance bone resorption by RANKL–RANK-mediated osteoclast activation, which in turn releases tumor growth factors stored in the bone matrix to promote tumor progression11. This bone environment-dependent concept had been demonstrated in primary bone tumors and metastasized bone tumors12. In addition, many studies have indicated that direct RANK signaling has complex effects on malignancies12. Activation of RANK can recruit TNF receptor-associated factors to transduce canonical downstream signaling, include NF-κB, phosphatidylinositol-4,5-bisphosphate 3-kinase, mitogen-activated kinase, and C-Jun N-terminal kinase8. The RANK-mediated signaling network is also associated with epithelial to mesenchymal transition, stemness, and metastatic genes8,13.
Santini et al.9 found that 75% of colorectal cancer (CRC) tissues were RANK-positive when detected using immunostaining. Studies had shown that RANKL–RANK-mediated bone resorption supported CRC dissemination in bone14, and blockage of RANKL–RANK signaling could inhibit CRC growth and the bone resorption caused by CRC in vivo15,16. However, the intrinsic role of RANK expressed on CRC remains unclear. In the current study, we identified that RANK expression was upregulated in CRC tissues and correlated with worse prognosis. Functional analyses demonstrated that RANK promoted the metastases of CRC cells both in vitro and in vivo. Mechanistically, RANK overexpression in CRC not only activated the canonical key downstream of RANK signaling, but also notably induced the Ca2+ oscillation dependent of phospholipase C-gamma (PLCγ)-mediated inositol-1,4,5-trisphosphate (IP3) receptor (IP3R) axis and stromal interaction molecule 1 (STIM1) to activate calcineurin/nuclear factor of activated T‑cells c1 (NFATC1)-tartrate-resistant acid phosphatase 5 (TRAP/ACP5) axis to promote metastasis. Our results highlighted the intrinsic role and mechanism of RANK in CRC metastasis.
Results
RANK was increased in CRC and associated with worse prognosis
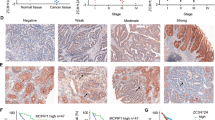
The GEPIA database showed that RANK mRNA levels were significantly increased in CRC tissues compared with those in normal colorectal tissues (Fig. 1a). Then, we used immunohistochemistry (IHC) staining to detect the expression of RANK in CRC. Representative images of different IHC staining grades of RANK are shown in Fig. 1b. RANK expression was upregulated in CRC tissues with different TNM stages compared with that in matched normal tissues distant (>10 cm) to the malignant lesion (Fig. 1c) or closely adjacent para-carcinoma tissues (Fig. 1d). As summarized in Fig. 1e, f, the proportion of high RANK expression significantly increased in CRC tissues than matched normal colonic epithelium (P < 0.0001) and increased gradually with the TNM stage (P < 0.0001). RANK expression was correlated prominently with worse clinicopathological parameters (Table 1). High RANK expression was associated markedly with decreased overall survival (OS; P = 0.0002) and disease-free survival (P = 0.0359; Fig. 1g, h). Moreover, univariate and multivariate survival analyses showed that RANK expression was an independent prognostic factor (Table 2) and recurrent factor (Table 3). Western blotting confirmed that RANK expression was upregulated in six (6/8, 75%) CRC samples compared with that in paired normal tissues (Fig. 1i). Taken together, RANK is frequently upregulated in CRC and is implicated in the pathogenesis or progression of CRC.
a RANK mRNA levels in CRC tissues and normal colorectal tissues were detected by analyzing the dataset GEPIA. *P < 0.05. b Representative images of different grades of RANK staining intensity were shown in CRC tissues. Staining results were classified as − (score 0), + (score 1–4), ++ (score 4–8), and +++ (score 9–12). IgG was the negative control for staining. NC negative control. c Representative RANK IHC staining in CRC tissues (stage I, stage II, stage III, and stage IV) and matched normal tissues far away (>10 cm) from the malignant lesion. d Characterization of RANK expression in CRC tissues and closely adjacent para-carcinoma tissues in eight patients (P1–P8). e Distribution of RANK expression in CRC tissues and matched normal tissues (P < 0.0001). f Percentage of RANK expression in CRC tumors within individual TNM stage (P < 0.0001). g Kaplan–Meier analysis of overall survival in CRC patients. h Kaplan–Meier analysis of disease-free survival in CRC patients. i Western blotting of RANK protein expressions in eight paired tumor and normal tissue samples. The tumor/normal ratios of RANK were quantified using the ImageJ software. Expression levels were normalized with GAPDH. T human CRC tissues, N paired normal colorectal tissues. Scales bars = 200 μm (100×), 100 μm (200×), and 50 μm (400×).
RANK promoted CRC migration and invasion in vitro and in vivo
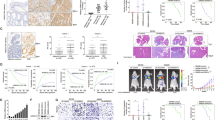
To investigate the role of RANK in CRC, we first screened the mRNA levels of RANK in CRC cell lines from the CCLE database (Fig. S1a). We then measured the RANK protein levels in seven cell lines with different mRNA levels (Fig. 2a). According to protein levels, we transfected control LV-vector and LV-RANK to construct RANK stably overexpressing SW480 (SW480RK) and Caco2 (Caco2RK) cells (Fig. 2b and Fig. S1b, c). Meanwhile, two independent shRNAs against RANK were transfected into HT29 cells to establish RANK stable knockdown cells (Fig. 2c and Fig. S1d). RANK overexpression or combined with 100 ng/ml of RANKL promoted the migration and invasion of SW480 and Caco2 cells (Fig. 2d, e), whereas knockdown of RANK inhibited migration and invasion of HT29 cells (Fig. 2f). However, RANK overexpression had no significant effect on the cell proliferation of CRC cell lines in either the absence or presence of RANKL (Fig. S1e, f). IHC staining showed that RANKL-positive cells exhibited the most similar distribution to CD3+CD25+FOXP3+ Treg cells in CRC tissues (Fig. S1g). The co-staining of CRC sections confirmed that RANKL and FOXP3 were colocalized (Fig. S1h) consistent with previous study17. We furthermore proved that RANKL was colocalized with CD4, but was not in relation to CD68+ macrophages, CD8+ killer T cells, and T helper (Th) cell subsets for analyzing their critical lineage determining transcription factors, including T-bet (Th1), GATA3 (Th2), and RORγT (Th17; Figs. S2 and S3). In addition, although the endogenous RANKL levels of CRC cells were very low (Fig. S4a), perturbation of endogenous RANKL by denosumab significantly attenuated migration and invasion of SW480RK and Caco2RK cells (Fig. S4b, c). These results indicated that baseline increase in migration and invasion was independent of exogenous RANKL, while low levels of endogenous RANKL are sufficient to elicit a metastatic phenotype in RANK-overexpressing CRC cells. In vivo experiments showed that mice injected with SW480RK cells developed remarkably greater local invasion and distant metastasis compared with that in the control group (Fig. 2g, h). Bioluminescent imaging and hematoxylin and eosin (HE) staining of resected livers revealed that SW40RK significantly increased liver metastatic nodules (Fig. 2i–k). These results proved that RANK functionally contributed to the migration and invasion of CRC cells in vitro and to hepatic metastasis in vivo.
a Protein expressions of RANK were detected by western blot analysis in seven CRC cell lines. b, c RANK stable overexpression or knockdown efficiency was confirmed by western blotting in CRC cells. d, e RANK overexpression promoted the migration and invasion of SW480 and Caco2 cells. And the addition of 100 ng/ml RANKL moderately increased the migration and invasion of RANK-overexpressing SW480 and Caco2 cells. Scales bars = 100 μm. f Knockdown of RANK inhibited the migration and invasion of HT29 cells. Scales bars = 100 μm. g Bioluminescent images of representative mice at week 15 after implantation. h The total tumor burden is quantified as total photons measured by bioluminescent technology. i Livers were excised for ex vivo bioluminescent imaging. j Representative results for staining of metastatic nodules (black arrows) in the livers. Scales bars = 500 μm (40×), 200 μm (100×). k The statistical analyses of metastatic nodules. Data are mean ± SD (n = 3; n = 6 mice/group for in vivo experiments). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
RANK regulated CRC migration and invasion by activating ACP5 expression
Increased basal levels of four canonical RANK-induced downstream pathways, phospho(p)-ERK1/2, p-P38, p-AKT, and p-NFκB-P65 with or without RANKL time-dependent stimulation were observed in SW480RK and Caco2RK cells compared with those in the parental cells (Fig. S5a, b). Furthermore, we investigated whether RANK expressed on CRC cells displayed similar downstream signaling to osteoclasts and secreted osteoclast-specific genes to degrade surrounding tissue for metastasis. Interestingly, quantitative reverse transcription polymerase chain reaction (qRT-PCR) showed that RANK overexpression significantly increased the mRNA levels of osteoclast marker genes, ACP5, CTSK (cathepsin K), MMP9 (matrix metalloproteinase 9), and VCAM1 (vascular cell adhesion molecule 1) in SW480 and Caco2 cells (Fig. S5c). Conversely, RANK depletion reduced the expression of these genes in HT29 cells (Fig. S5d). Moreover, ACP5 mRNA expressions were significantly increased in CRC tissues compared with that in normal colorectal tissues (Fig. S5e), and high ACP5 expression decreased OS from online databases (Fig. S5f). These results were confirmed by related experimental analysis of patients with CRC18. Thus, we were prompted to explore whether RANK overexpression promoted CRC migration and invasion depending on ACP5. Western blotting showed that RANK overexpression significantly increased ACP5 levels (Fig. 3a), whereas RANK depletion downregulated ACP5 levels in CRC cells (Fig. 3b). Silencing of ACP5 in SW480RK and Caco2RK cells attenuated RANK-induced migration and invasion (Fig. 3c–e). Overexpression of ACP5 could also rescue the reduction of HT29 cell migration and invasion induced by RANK silencing (Fig. 3f–h). Immunofluorescence confirmed that ACP5 distribution within the tumor and stroma was more obvious in CRC tissues with high RANK expression than in those with low RANK expression (Fig. 3i). These results indicated that ACP5 was critical for RANK-induced promotion of CRC migration and invasion.
a, b Western blot analysis of upregulation of ACP5 protein in RANK-overexpressing SW480 and Caco2 cells, and downregulation of ACP5 protein in RANK knockdown HT29 cells. c, f Western blotting showed that successful decreased ACP5 protein in RANK overexpression CRC cells transfected with siRNA-ACP5 and increased ACP5 protein by transfection with pFV-ACP5 plasmid in RANK knockdown CRC cells. d, e Knockdown of ACP5 inhibited RANK-induced migration and invasion in SW480RK and Caco2RK cells. Scales bars = 100 μm. g, h ACP5 overexpression increased migration and invasion of RANK-silencing HT29 cells. Scales bars = 100 μm. i Representative immunofluorescence of ACP5 distribution in high and low RANK expression CRC tissues. Nuclei (blue) were counterstained with DAPI. The white arrowheads indicate ACP5-positive cells are distributed within the tumor and stroma with high RANK expression. + (score 1–4), +++ (score 9–12). Scales bars = 10 μm. Data are mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
RANK regulated ACP5 through the calcineurin/NFATC1 axis
Accumulating evidence suggests that some osteoclast-specific genes, including ACP5, are directly regulated by NFATC1 (refs. 19,20). We next explored whether NFATC1 was involved in a downstream mechanism of RANK to regulate ACP5 in CRC metastasis. High NFATC1 expression was associated with worse OS or relapse-free survival in patients with CRC by analyzing the online databases (Fig. S6a–d). In addition, RANK mRNA expressions exhibited a strong positive correlation with NFATC1 in patients with CRC in the indicated databases (Fig. S6e–g). QRT-PCR confirmed that the NFATC1 mRNA levels were increased in SW480RK and Caco2RK cells, and decreased in RANK knockdown HT29 cells (Fig. S6h, i). NFATC1 undergoes efficient cytoplasmic dephosphorylation for nuclear translocation and facilitates its binding to downstream targets21. Western blotting demonstrated that RANK overexpression induced dephosphorylation of cytoplasmic NFATC1 and its nuclear translocation, while knockdown of RANK denoted the opposite results in CRC cells (Fig. 4a, b). Immunofluorescence also confirmed that RANK overexpression enriched NFATC1 levels in the nucleus, whereas RANK silencing had the opposite effect (Fig. 4c, d). Furthermore, increased nuclear NFATC1 levels were observed in high RANK expression CRC tissues compared with those in matched normal tissues and tumor tissues with negative RANK expression, as revealed by immunofluorescence staining (Fig. 4e). We then investigated whether RANK upregulated ACP5 expression to promote metastasis depending on NFATC1 in CRC. The online datasets showed that NFATC1 mRNA expressions directly correlated with ACP5 expression in patients with CRC (Fig. 4f–h). In addition, CHIP-seq datasets from ENCODE22 showed that NFATC1 and other transcription factors, such as MITF, PU.1, and AP-1 were all enriched on the ACP5 promoter (Fig. 4i). These transcription factors have been proven to interact with the ACP5 promoter and activate its expression19,20,23,24. In our study, we confirmed that silencing of NFATC1 in SW480RK or Caco2RK cells decreased ACP5 expression significantly (Fig. 4j), and attenuated RANK-induced migration and invasion (Fig. 4k, l). These results revealed that NFATC1, activated by RANK, specifically occupied the ACP5 promoter to regulate the migration and invasion of CRC cells.
a, b Western blotting analyzed the protein levels of phosphorylated NFATC1 and NFATC1 in cytoplasm, and nucleus of RANK-overexpressing or knockdown CRC cells. GAPDH served as the cytoplasmic control and lamin B1 as the nuclear protein loading control. c Immunofluorescence staining of subcellular localization of NFATC1 in RANK overexpression and control cells with or without treatment of 10 μg/ml cyclosporin A (CsA). Scales bars = 20 μm. d Representative immunofluorescence of subcellular localization of NFATC1 in RANK-depleted HT29 cells. Scales bars = 20 μm. e The NFATC1 subcellular localization in matched normal tissues, negative and high RANK expression of CRC tissues by immunofluorescence staining. DAPI (nuclei, blue). RANK is visualized in the membranes and in the cytoplasm surrounding the nucleus of the NFATC1 in CRC tissues (white arrowheads). − (score 0), +++ (score 9–12). N paired normal colorectal tissues. Scales bars = 10 μm. f–h Correlation analysis between mRNA levels of NFATC1 and ACP5 from indicated online datasets. i ChIP-seq data showed a UCSC Genome Browser screenshot of the ACP5 promoter region for NFATC1 and SPI1 in GM12878 cells and MITF, SPI1, and JUN in K562 cells from ENCODE. j NFATC1 knockdown reversed RANK-induced ACP5 expression. k, l Silencing of NFATC1 decreased RANK-induced migration and invasion in SW480 and Caco2 cells. Scales bars = 100 μm. Data are mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Notably, calcineurin, as a direct upstream regulator of NFATC1, dephosphorylates NFATC1 and initiates its nuclear translocation21. Thus, we further tested whether the inhibition of calcineurin had an effect on RANK-induced migration and invasion in CRC. Treatment with the calcineurin inhibitor cyclosporin A (CsA) gradually attenuated the migration and invasion of SW480RK and Caco2RK cells at 1, 5, and 10 μg/ml (Fig. 5a, b). Immunofluorescence and western blotting and showed that 10 μg/ml CsA decreased NFATC1 nuclear translocation significantly in SW480RK and Caco2RK cells (Figs. 4c and 5c). In addition, 10 μg/ml CsA reversed the upregulation of ACP5 protein levels in SW480RK and Caco2RK cells (Fig. 5d). Collectively, our results demonstrated that RANK overexpression activated ACP5 expression through calcineurin/NFATC1 signaling, which eventually induced metastasis in CRC.
a, b Cyclosporin A (CsA) gradually attenuated RANK-induced migration and invasion in SW480 and Caco2 cells at 1, 5, and 10 μg/ml. Scales bars = 100 μm. c A total of 10 μg/ml CsA significantly decreased the nuclear translocation of NFATC1 in SW480RK and Caco2RK cells analyzed by western blotting. d Western blotting showed that 10 μg/ml CsA reversed the ACP5 levels in RANK-overexpressing CRC cells. Data are mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
RANK activated the calcineurin/NFATC1 axis via Ca2+ oscillation
Ca2+ oscillation is essential to maintain NFATC1 in the nucleus and enables transcriptional activation of NFATC1 (ref. 25). To explore the impact of Ca2+ signaling on RANK-induced NFATC1 activation, we first evaluated cytoplasmic Ca2+ oscillation in CRC cells. Calcium-flux measurement showed that overexpression of RANK in SW480, and Caco2 cells increased cytoplasmic Ca2+ levels and Ca2+ influx significantly (Fig. 6a). The opposite results were observed in RANK knockdown HT29 cells (Fig. 6b). Store-operated Ca2+ entry (SOCE) is a major mechanism to increase cytosolic Ca2+ influx and has been reported to be associated with cancer malignancy in CRC cells26,27. The STIM1, ORAI calcium release-activated calcium modulator 1 (ORAI1), and canonical transient receptor potential channel 1 (TRPC1) protein families are the main modulators of SOCE27,28. We found that the mRNA levels of STIM1, ORAI1, and TRPC1 were upregulated significantly in SW480RK and Caco2RK cells, and downregulated in RANK knockdown HT29 cells (Fig. S7a, b). STIM1, as the endoplasmic reticulum (ER) Ca2+ sensor for SOCE, migrates from the ER to the plasma membrane to activate Ca2+ influx by SOCE29,30. STIM1 overexpression promotes CRC metastasis, and is associated with progression and poor prognosis in CRC31,32. Thus, we analyzed whether STIM1 was a downstream factor of RANK to regulate the calcineurin/NFATC1 axis. We found that the mRNA expressions of RANK correlated positively with STIM1 in patients with CRC by analyzing database GEPIA (Fig. S7c). Western blotting confirmed that RANK overexpression induced STIM1 upregulation and RANK knockdown reduced STIM1 expression in CRC cells (Fig. 6c, d). Immunofluorescence staining further confirmed that strong cytoplasmic STIM1 staining was detected in high RANK expression CRC tissues, while weak STIM1 staining was observed in matched normal epithelial tissues with low RANK expression (Fig. 6e). Moreover, the online datasets showed that the STIM1 mRNA levels correlated positively with NFATC1 and ACP5 levels in patients with CRC (Fig. 6f–h and Fig. S7d–f). Consistently, silencing of STIM1 by small interfering RNA (siRNA) transfection inhibited the reduction of cytoplasmic phosphorylated NFATC1, and ACP5 activation induced by RANK overexpression (Fig. 6i). The rescue experiment revealed that silencing STIM1 downregulated the migration and invasion of SW480RK and Caco2RK cells (Fig. 6j, k). These results indicated that the STIM1-Ca2+ signaling pathway is involved in the RANK-induced activation of the calcineurin/NFATC1-ACP5 axis and metastasis.
a, b The calcium-flux analysis in response to ionomycin in CRC cells with RANK overexpression or knockdown. Besides, the effect of 100 μM 2APB on RANK-overexpressing SW480 and Caco2 cells were analyzed. c, d Western blotting showed that STIM1 protein levels were regulated by RANK in CRC cells. e STIM1 was co-stained with RANK in CRC tissue and paired normal colon tissues by immunofluorescence. The white arrowheads indicate that the overlap of RANK-positive cells and STIM1-positive CRC tissues. DAPI staining for nuclei. Scales bars = 50 μm. T human CRC tissues, N paired normal colorectal tissues. f–h The mRNA levels of STIM1 were positively correlated with NFATC1 in CRC assessed by online databases. i Silence of STIM1 reversed RANK-induced ACP5 expression activation and increased phosphorylated NFATC1. j, k Silence of STIM1 rescued the migration and invasion of RANK-overexpressing SW480 and Caco2 cells. Scales bars = 100 μm. Data are mean ± SD (n = 3). **P < 0.01, ***P < 0.001, ****P < 0.0001.
PLCγ-mediated IP3 production and subsequent activation of ER Ca2+ release play a substantial role in osteoclast differentiation33. The indicated online datasets showed that mRNA levels of RANK were positively correlated with IP3R and PLCγ in patients with CRC (Fig. S8a–d). We supposed that RANK upregulated cytosolic Ca2+ concentration in CRC through ER Ca2+ release via PLCγ-mediated IP3 production, as well as by Ca2+ influx dependent on STIM1. To validate this, the effect of the IP3R antagonist, 2-aminoethoxydiphenyl-borate (2APB) on Ca2+ oscillation was assessed. We found that pretreatment with 100 μM 2APB could attenuate the cytosol Ca2+ concentration at both basal and RANK overexpression stimulatory level in CRC cells (Fig. 6a). Western blotting showed that 2APB prevented NFATC1 cytoplasmic dephosphorylation and nuclear translocation in SW480RK and Caco2RK cells (Fig. 7a). The 2APB-induced reversal of significantly nuclear translocation in RANK-overexpressing CRC cells was also confirmed by immunofluorescence (Fig. 7b). Furthermore, 2APB could rescue RANK-induced migration and invasion in SW480 and Caco2 cells (Fig. 7c, d). The mRNA expressions of IP3R were found to have positive correlation for PLCγ in patients with CRC by analyzing the online datasets (Fig. 7e–g). Next, we examined the effect of inhibition of PLCγ on RANK-induced activity of CRC cells. Western blotting showed that the PLCγ inhibitor U73122 reversed NFATC1 nuclear translocation, and ACP5 upregulation in SW480RK and Caco2RK cells (Fig. 7h). Notably, U73122 decreased the protein levels of STIM1 significantly in RANK-overexpressing CRC cells, indicating that PLCγ-mediated IP3 also regulates the activity of SOCE (Fig. 7h). The online databases also confirmed that mRNA expressions of IP3R correlated positively with STIM1 in patients with CRC (Fig. S8e–g). Furthermore, U73122 significantly attenuated the migration and invasion of SW480RK and Caco2RK cells (Fig. 7i, j). The above results revealed that RANK induced the calcineurin/NFATC1 axis by activation of PLCγ-IP3-STIM1-mediated ER Ca2+ release and Ca2+ influx.
a Western blotting analyzed the protein levels of NFATC1 and phosphorylated NFATC1 in nuclear and cytoplasm of 100 μM 2APB-treating CRC cells. b Immunofluorescence staining showed nucleocytoplasmic localization of NFATC1 in CRC cells treated by 100 μM 2APB. Blue represents DAPI staining. Scales bars = 20 μm. c, d A total of 100 μM 2APB rescued RANK-mediated migration and invasion of SW480 and Caco2 cells. Scales bars = 100 μm. e–g Scatter plots showed the significant positive relationship between mRNA expressions of IP3R and PLCγ obtained by online datasets. PLCG1, PLCγ. ITPR3, IP3R. h A total of 10 μM U73122 rescued the protein levels of STIM1, ACP5, and the phosphorylation level of NFATC1 in SW480RK and Caco2RK cells. i, j Representative images of CRC cells treated by 10 μM U73122 subjected to the transwell migration and invasion assays. Scales bars = 100 μm. Data are mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
Approximately 25% of patients with CRC present with metastases at initial diagnosis and almost 50% ultimately develop metastases, leading to the high mortality rates observed in CRC34. Despite improvements in therapeutic early-stage CRC over past decades, no effective therapy is currently available for advanced or metastatic CRC for poor understanding of the mechanisms underlying CRC metastasis35. In the present study, we found higher RANK expression in CRC tissues compared with that in matched normal tissues, which correlated with worse prognosis. Furthermore, RANK expressed in CRC-activated PLCγ-mediated IP3 signaling to evoke Ca2+ release from the ER, and upregulated STIM1 to promote Ca2+ influx through SOCE, which cooperate to generate Ca2+ oscillation. In addition, STIM1 was also regulated by PLCγ induction. RANK-mediated intracellular Ca2+ mobilization subsequently stimulated calcineurin to dephosphorylate NFATC1 and induce NFATC1 nuclear translocation. In the nucleus, NFATC1 transcriptionally upregulates osteoclast-specific gene ACP5 to promote the migration and invasion of CRC (Fig. 8), indicating that RANK might serve as a potential therapeutic target.
Active RANK expression allows for Ca2+ release from ER dependent on PLCγ-mediated IP3R and Ca2+ influx from the extracellular milieu through STIM1-regulated SOCE. Besides, PLCγ-mediated IP3R axis act upstream of STIM1 to coactivate Ca2+ oscillation. The Ca2+ oscillation in the cytoplasm leads to calcium-dependent calcineurin activation and NFATC1 transcription of osteoclast-specific gene, ACP5, which promotes CRC metastasis. PLCγ phospholipase C γ, IP3R inositol-1,4,5-trisphosphate receptor, SOCE store-operated calcium entry, STIM stromal interaction molecule, TRPC transient receptor potential channel, NFATC1 nuclear factor of activated T cells c1, CsA cyclosporine A, 2APB 2-aminoethoxydiphenyl-borate, ACP5 tartrate-resistant acid phosphatase 5, ER endoplasmic reticulum.
To the best of our knowledge, this study is the first to report functional RANK expression in CRC. Jones et al.4 first mentioned the relationship between RANK and metastasis in CRC cells. Their study showed that the human CRC cell lines SW480 and Colo205 had no detectable levels of RANK. These CRC cells failed to migrate when induced by RANKL and metastasized into the bones after intracardiac injection. Thus, for a long time afterward, research associated with RANK in cancer mainly focused on breast and prostate cancer, which frequently develop bone metastasis. RANK also has a role in other cancers, such as osteosarcoma36 and melanoma37; however, there have been few studies in CRC. This might be explained by the fact that RANK is related to the bone environment, whereas CRC mainly develops liver metastasis, not bone metastasis, like breast and prostate cancer. Although one study mentioned that 75% of CRC tissues were RANK-positive9 and scattered reports showed that the role of RANK in CRC was related to the bone environment or osteoclast activity14,15,16, the direct function of RANK in CRC is still unknown. In this work, we clearly demonstrated positive immunostaining of RANK in CRC tissues, and confirmed high RANK expression in the CRC cell line HT29 and its low expression in SW480 and Caco2 cells. These results were similar to those reported by previous studies4,9. In addition, we found that RANK expression correlated negatively with the prognosis of patients with CRC. Gain and loss of function studies showed that RANK promoted the migration and invasion of CRC cells. Overexpression of RANK in CRC cells resulted in constitutive activation of the canonical downstream signaling pathway (p-P65, p-P38, p-AKT, and p-ERK). Moreover, presence of RANKL significantly increased the basal levels of these RANK downstream targets. Thus, our results clearly demonstrated that overexpression of RANK led to RANKL-dependent and RANKL-independent activation of downstream pathways, as described in other cancers4,13. Interestingly, we found that tumor-infiltrating CD25+FOXP3+Treg cells were the major producers of RANKL within the microenvironment of RANK-expressing CRC, which may provide a reference for the treatment of CRC metastasis. Taken together, our study proved that RANK expressed in CRC is functional.
Overexpression of RANK promoted CRC metastasis by activating the Ca2+-calcineurin/NFATC1-ACP5 axis. Previous studies have proven that osteoclast-associated signaling molecules promote CRC metastatic capacity, notably the transcription factor NFATC1 and its regulation by calcineurin38,39. Questions concerning which factor activates the calcineurin/NFATC1 axis in CRC and how calcineurin/NFATC1 regulates CRC metastasis remain unanswered. In the present study, we found that PLCγ-IP3-mediated ER Ca2+ release and STIM1-regulated Ca2+ influx through SOCE acted together to activate calcineurin/NFATC1 in RANK-overexpressing CRC cells. Moreover, we found that STIM1 was also regulated by PLCγ, indicating that PLCγ-IP3 signaling and STIM1 were involved closely in the Ca2+ oscillation induced by RANK in CRC. After being activated, calcineurin enhances NFATC1 activity by dephosphorylating it, thus increasing NFATC1’s nuclear translocation. NFATC1 further activates the transcription of the osteoclast-specific gene, ACP5. ACP5 is a metalloenzyme of the acid phosphoprotein phosphatase family40 and the major function of ACP5 in bone resorption is the catabolic degradation of bone matrix phosphoproteins. ACP5 has been recommended as a serum marker for bone resorptive activity in pathological states, such as osteoporosis and notably, bone metastasis of cancers41,42. Previous studies revealed that high ACP5 expression correlated with reduced survival and increased metastasis in various cancers43,44,45. In addition, ACP5 has been proven to aggravate the proliferation and invasion of CRC cells and might serve as an indicator for poor prognosis in CRC18. Consistently, our research also showed that RANK-induced ACP5 affected the NFATC1 pathway to promote the migration and invasion of CRC cells significantly. These results support a role for RANK in the regulation of CRC metastasis and provide novel insights into the molecular pathways linking RANK expressed in CRC to osteoclast differentiation signaling. Therefore, inhibition of osteoclastogenesis signaling not only interrupts the vicious cycle between bone and CRC, but also more importantly, it directly decreases the metastatic ability of CRC with high RANK expression. Moreover, these results implied that OPG and denosumab, the drugs that block RANKL–RANK interaction, are not sufficient to suppress CRC bone metastasis and skeletal-related events (SREs). Drugs targeting the Ca2+-calcineurin/NFATC1-ACP5 axis in RANK-expressing CRC might be needed, such as the inhibitors CsA, 2APB, and U73122 used in the present study.
There were several limitations of this study. Although CRC metastasis was independent of exogenous RANKL, the presence of RANKL could enhance RANK-overexpressing CRC metastasis and RANK signaling activation. Our IHC staining found that RANKL might be derived from tumor-infiltrating CD25+FOXP3+ Treg cells. Further experiments are needed to verify the exact source of RANKL to better determine the role of the RANK–RANKL system in CRC. In addition, it remains to be determined how RANK initially activates PLCγ to induce the Ca2+-calcineurin/NFATC1 axis, which will require further mechanistic experiments.
In conclusion, our findings demonstrated that RANK is upregulated in CRC and correlates with poor outcomes in patients with CRC. In addition, we determined a novel role of RANK expression in promoting CRC metastasis in vitro and in vivo. Mechanistically, in CRC, RANK induces PLCγ-IP3-STIM1 signaling-mediated Ca2+ oscillation and further activated the calcineurin/NFATC1-ACP5 pathway to regulate CRC metastasis. This axis linking RANK to osteoclast-specific gene ACP5 might provide promising targets to develop antimetastatic agents to treat patients with CRC.
Materials and methods
Patients and tissue specimens
A total of 203 pairs of CRC and nontumor colonic epithelium paraffin-embedded tissues were obtained from operated patients at The First Affiliated Hospital, Sun Yat-sen University (Guangzhou, China) from 2008 to 2011. Follow-ups of these 203 patients ended on December 1st, 2019. The follow-up duration ranged from 2 to 130 months. The fresh CRC and matched normal tissues were collected from operated patients at The Seven Affiliated Hospital, Sun Yat-sen University (Shenzhen, China). Patients who underwent preoperative chemotherapy and/or radiotherapy were excluded. Each patient provided written informed consent. The use of clinical samples was approved by the Ethics Committee of Sun Yat-sen University.
Antibodies and reagents
Primary antibodies used in this study included: anti-RANK (ab13918), anti-FOXP3 (ab20034), anti-CD3 (ab16669), anti-CD19 (ab134114), anti-rabbit RANKL (ab9957), anti-CD25 (ab128955), anti-CD4 (ab133616), anti-CD8 (ab4055), and anti-CD68 (ab201340) from Abcam; anti-ERK (#9102), anti-p-ERK (Thr202/Tyr204; #9101), anti-P38 (#9212), anti-p-P38 (Thr180/Tyr182; #9211), anti-P65 (#4764), anti-p-P65 (Ser536; #3033), anti-AKT (#9272), and anti-p-AKT (Ser473; #9101) from CST; anti-IgG isotype control (10500 C) from Thermo; anti-GAPDH (60004-1-lg), anti-STIM1 (11565-1-AP), GATA3 (66400-1-lg), and anti-mouse RANKL (66610-1-lg) from Proteintech, Wuhan, China; anti-ACP5 (DF6989), anti-rabbit RORγ (DF3196), and anti-T-bet (DF7759) from Affinity Biosciences, OH, USA; anti-mouse RORγ (sc-365476) from Santa Cruz, CA, USA; and anti-lamin B1 from (AF1408, Beyotime, Beijing, China). The reagents included: CsA, U73122, 2APB, and ionomycin from MCE; RANKL, EGF, and bFGF from PeproTech; and denosumab from TheraMabs (Shanghai, China).
Immunohistochemistry
After routine deparaffinization and rehydration, treatment with hydrogen peroxide, antigen retrieval, and blocking with goat serum, the tissue sections were incubated with primary antibodies overnight at 4 °C. Rabbit IgG was used as negative control. All antibodies were diluted according to the instructions. Next, the slides were incubated with secondary antibody, stained with diaminobenzidine tetrahydrochloride (DAKO, Carpinteria, CA), and counterstained with hematoxylin. The IHC staining was analyzed by two independent pathologists who were blinded to the patient’s clinical parameters. The staining intensity was documented as 0 (no staining), 1 (weak immunoreactivity), 2 (moderate immunoreactivity), and 3 (strong immunoreactivity). The percentage of immunoreactive cells was scored as 0 (no positive cells), 1 (<20%), 2 (20–50%), 3 (51–75%), and 4 (>75%). The final score was determined by multiplying the intensity and the percentage of immunoreactive cells. Depending on the final score, the staining results were graded as − (score 0), + (score 1–4), ++ (score 5–8), and +++ (score 9–12). The grade was furthermore defined as low expression (−, +) and high expression (++, +++)46,47.
Cell lines and culture conditions
Human CRC cell lines SW480, Caco2, HT29, DLD1, HCT116, LS174T, and LOVO were purchased from the American Type Culture Collection. All cells were authenticated and free of mycoplasma. HT29 were cultured in RPMI 1640 Medium (Gibco), and the other cell lines were maintained in Dulbecco’s modified Eagle’s medium (Gibco) at 37 °C with 5% CO2. Medium was added with 10% fetal bovine serum (FBS; Gibco) for cell culture.
Cell lines transfection
For transient transfection, two independent siRNA duplexes against STIM1, ACP5, and control siRNA, overexpression plasmids including pFV155-GFP-puro-ACP5 and control vector were introduced into cells using Lipofectamine 3000 reagent (Invitrogen), according to the manufacturer’s instructions. For stable overexpression and knockdown transfection, 293T cells were transfected with pFV155-GFP-puro-RANK, pLKO.1-puro-RANK-target shRNA, PTSB-NEO-NFATC1-target shRNA, and their empty vectors using package plasmids and polyethyleneimine (Polysciences). Virus particles were harvested 48 h after transfection and used for cell infection with polybrene (Sigma). Puromycin (Sigma) and G418 (Sigma) were used for puro-resistance and NEO-resistance plasmids, respectively. All siRNA duplexes, shRNA constructs, and overexpressing plasmids were from Transheep (Shanghai, China). The siRNA and shRNA sequences are listed in Supplementary Tables S1 and S2.
Western blot analysis
Briefly, proteins from total cell lysates (20 μg) were separated by SDS–PAGE, transferred onto polyvinylidene difluoride membranes (Millipore), and immunoblotted with the indicated antibodies, followed by peroxidase-conjugated anti-mouse or rabbit IgG. Anti-GAPDH antibody was diluted at 1:10,000 and other primary antibodies were 1:1000. Nuclear and Cytoplasmic Protein Extraction kit (Beyotime, Beijing, China) was used to separate the nuclear and cytoplasmic protein, according to the manufacturer’s protocol. Membranes were finally infiltrated by an enhanced chemiluminescence reagent and visualized using Chemiluminescence imaging Systems (Bio-Rad).
Quantitative reverse transcription polymerase chain reaction
Total RNA was extracted from cells using AG RNAex Pro Reagent (AG21102, ACCURATE BIOTECHNOLOGY, Hunan, China), according to the manufacturer’s protocol. Reverse transcription was performed using the Evo M-MLV RT Premix (AG11706, ACCURATE BIOTECHNOLOGY, Hunan, China). The qRT-PCR assay was performed using SYBR® Green Premix Pro Taq HS qPCR Kit (AG11701, ACCURATE BIOTECHNOLOGY, Hunan, China) in the CFX96 Touch Real-Time PCR system (CFX96, BIO-RAD Laboratories, Hercules, USA). GAPDH was used as an endogenous reference to normalize RNA expression. The primer sequences used are listed in Supplementary Table S3.
Migration and invasion assays
A total of 2 × 105 cells in serum-free medium were seeded into the upper chamber of inserts (8 μm pore size, BD Biosciences, USA) with or without diluted Matrigel (Corning, USA) for migration and invasion in 24-well plates. Besides, the indicated concentration of chemicals used in this study was added into the upper chambers to evaluate their effect. Medium supplemented plus 20% FBS was used as a chemoattractant in the lower chambers. After 60-h incubation, the cells on the lower membrane were fixed and stained with crystal violet. The staining cells were randomly imaged in eight different fields with a microscope.
Cell proliferation assay
A total of 1000 cells were inoculated into 96-well plates with complete medium. Four consecutive days after initial planting, cells were incubated in the medium with 10% Cell Counting Kit-8 (Dojindo, Japan) for 2 h at 37 °C in dark. The cell absorbance was then detected at 450 nm with a microplate reader (Synergy H1M, BioTek).
Immunofluorescence
Cells were seeded onto sterile slides into 24-well culture plates. After reached a suitable confluence, cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton/PBS, and blocked with goat serum. The methods for tissue slices before primary antibody incubation were the same as IHC.
Next, cells were incubated with indicated primary antibody overnight at 4 °C. For double immunofluorescence of tissue slices, both primary antibodies were mixed and incubated. All antibodies were used according to the instructions. On the second day, cells or slices were further incubated with Alexa Fluor 488 goat anti-mouse IgG (A-11001, Thermo) or Alexa Fluor 594 goat anti-rabbit IgG (A-11012, Thermo), stained with DAPI (Sigma) label nuclei, and finally observed under Leica fluorescence or Zeiss confocal microscopy.
Animal experiments
Female 6-week-old BABL/c nude mice were purchased from Beijing Vital River Laboratory Animal Technology Company (Beijing, China). All animal experiments were approved by the Sun Yat-sen University Institutional Animal Care and Use Committee. The mice were randomly allocated into two groups (n = 6) and received intrasplenic transplantation to study liver metastasis. For the spleen injection, the mice were anesthetized with isoflurane and laparotomized to expose the spleen. A total of 1.5 × 106 SW480-Luc/Control or SW480-Luc/RANK cells in 50 μl PBS were slowly injected into the spleen using an insulin syringe. The spleen was then replaced into the abdomen cavity and the abdominal wall was sutured. Liver metastasis was determined by bioluminescence imaging using the IVIS Imaging System (Xenogen). After 15 weeks, the mice were euthanized and the livers were removed for bioluminescence imaging and HE staining to confirm metastatic foci. The statistical analyses were performed by researchers blinded to experiment design.
Calcium-flux measurements
Cells were trypsinized and incubated with 10 μM Calbryte™ 630 AM Esters (AAT Bioquest) for 1 h at room temperature, according to instruction. Then, cells were switched to Hanks and Hepes buffer containing 2 mM Ca2+, and subjected to FACS analysis by a CytoFLEX flow cytometer (Beckman) to measure fluorescence intensity. During the FACS analysis, cells were stimulated with ionomycin (2 μg/ml). The results were further quantitatively analyzed by FlowJo (v10) software.
Bioinformation analysis
The gene expression data and Kaplan–Meier survival plots for CRC patients were appraised by online database GEPIA (Gene Expression Profiling Interactive Analysis, http://gepia.cancer-pku.cn/), UALCAN (http://ualcan.path.uab.edu/analysis.html), and Prognostic Database (http://genomics.jefferson.edu/proggene/). The mRNA expressions of RANK in CRC cell lines were analyzed using the CCLE database (https://portals.broadinstitute.org/ccle/home). Correlation analysis between gene expression in tissues of CRC patients was computed from cBioPortal (www.cbioportal.org), TIMER (Tumor IMmune Estimation Resource, https://cistrome.shinyapps.io/timer/), and GEPIA. CHIP-seq datasets were collected from the ENCODE Project Consortium (https://www.encodeproject.org/).
Statistical analysis
Statistical analysis was performed using SPSS 20.0 and GraphPad Prism 6.0. The indicated protein levels to GAPDH are quantified using ImageJ software. Data were compared by Student’s t test or one-way ANOVA test. The χ2 test and Fisher’s exact test were used to analyze the relationship between RANK expression and clinical characteristics. Survival curves were plotted with the Kaplan–Meier method with the log-rank test. Cox regression was employed for univariate and multivariate analyses. Variables with a P value < 0.05 in univariate analysis were included in multivariate analysis. All data are presented as the mean ± SD. P value < 0.05 was considered statistically significant.
References
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Rao, S., Cronin, S. J. F., Sigl, V. & Penninger, J. M. RANKL and RANK: from mammalian physiology to cancer treatment. Trends Cell Biol. 28, 213–223 (2018).
Walsh, M. C. & Choi, Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front. Immunol. 5, 511 (2014).
Jones, D. H. et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440, 692–696 (2006).
Luo, J. L. et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature 446, 690–694 (2007).
Tan, W. et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470, 548–553 (2011).
Sisay, M., Mengistu, G. & Edessa, D. The RANK/RANKL/OPG system in tumorigenesis and metastasis of cancer stem cell: potential targets for anticancer therapy. OncoTargets Ther. 10, 3801–3810 (2017).
Renema, N., Navet, B., Heymann, M. F., Lezot, F. & Heymann, D. RANK-RANKL signalling in cancer. Biosci. Rep. 36, e00366 (2016).
Santini, D. et al. Expression pattern of receptor activator of NFκB (RANK) in a series of primary solid tumors and related bone metastases. J. Cell. Physiol. 226, 780–784 (2011).
Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 8, 98–101 (1989).
Maurizi, A. & Rucci, N. The osteoclast in bone metastasis: player and target. Cancers 10, 218 (2018).
Cheng, M. L. & Fong, L. Effects of RANKL-targeted therapy in immunity and cancer. Front. Oncol. 3, 329 (2014).
Palafox, M. et al. RANK induces epithelial-mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis. Cancer Res. 72, 2879–2888 (2012).
Reynaud, C. et al. Lysyl oxidase is a strong determinant of tumor cell colonization in bone. Cancer Res. 77, 268–278 (2017).
Wei, Y. et al. Heterologous expression, purification and function of the extracellular domain of human RANK. BMC Biotechnol. 17, 87 (2017).
Morony, S. et al. The inhibition of RANKL causes greater suppression of bone resorption and hypercalcemia compared with bisphosphonates in two models of humoral hypercalcemia of malignancy. Endocrinology 146, 3235–3243 (2005).
Tan, W. et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470, 548–553 (2011).
Bian, Z. Q. et al. Overexpressed ACP5 has prognostic value in colorectal cancer and promotes cell proliferation and tumorigenesis via FAK/PI3K/AKT signaling pathway. Am. J. Cancer Res. 9, 22–35 (2019).
Takayanagi, H. et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3, 889–901 (2002).
Matsumoto, M. et al. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J. Biol. Chem. 279, 45969–45979 (2004).
Crabtree, G. R. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell 96, 611–614 (1999).
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Partington, G. A., Fuller, K., Chambers, T. J. & Pondel, M. Mitf-PU.1 interactions with the tartrate-resistant acid phosphatase gene promoter during osteoclast differentiation. Bone 34, 237–245 (2004).
Luchin, A. et al. Genetic and physical interactions between Microphthalmia transcription factor and PU.1 are necessary for osteoclast gene expression and differentiation. J. Biol. Chem. 276, 36703–36710 (2001).
Negishi-Koga, T. & Takayanagi, H. Ca2+−NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol. Rev. 231, 241–256 (2009).
Sobradillo, D. et al. A reciprocal shift in transient receptor potential channel 1 (TRPC1) and stromal interaction molecule 2 (STIM2) contributes to Ca2+ remodeling and cancer hallmarks in colorectal carcinoma cells. J. Biol. Chem. 289, 28765–28782 (2014).
Villalobos, C., Hernández-Morales, M., Gutiérrez, L. G. & Núñez, L. TRPC1 and ORAI1 channels in colon cancer. Cell Calcium 81, 59–66 (2019).
Jardin, I., Lopez, J. J., Salido, G. M. & Rosado, J. A. Store-operated Ca entry in breast cancer cells: remodeling and functional role. Int. J. Mol. Sci. 19, 4053 (2018).
Hogan, P. G., Lewis, R. S. & Rao, A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 28, 491–533 (2010).
Zhang, S. L. et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905 (2005).
Zhang, Z. et al. STIM1, a direct target of microRNA-185, promotes tumor metastasis and is associated with poor prognosis in colorectal cancer. Oncogene 34, 4808–4820 (2015).
Wang, J. Y. et al. STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene 34, 4358–4367 (2015).
Kuroda, Y., Hisatsune, C., Nakamura, T., Matsuo, K. & Mikoshiba, K. Osteoblasts induce Ca2+ oscillation-independent NFATc1 activation during osteoclastogenesis. Proc. Natl Acad. Sci. USA 105, 8643–8648 (2008).
Van Cutsem, E., Cervantes, A., Nordlinger, B. & Arnold, D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 25, iii1–iii9 (2014).
Tauriello, D. V. F., Calon, A., Lonardo, E. & Batlle, E. Determinants of metastatic competency in colorectal cancer. Mol. Oncol. 11, 97–119 (2017).
Beristain, A. G., Narala, S. R., Di Grappa, M. A. & Khokha, R. Homotypic RANK signaling differentially regulates proliferation, motility and cell survival in osteosarcoma and mammary epithelial cells. J. Cell Sci. 125, 943–955 (2012).
Kupas, V. et al. RANK is expressed in metastatic melanoma and highly upregulated on melanoma-initiating cells. J. Invest. Dermatol. 131, 944–955 (2011).
Tripathi, M. K. et al. Nuclear factor of activated T-cell activity is associated with metastatic capacity in colon cancer. Cancer Res. 74, 6947–6957 (2014).
Wang, G. et al. The critical role of calcineurin/NFAT (C/N) pathways and effective antitumor prospect for colorectal cancers. J. Cell. Biochem. 120, 19254–19273 (2019).
Oddie, G. W. et al. Structure, function, and regulation of tartrate-resistant acid phosphatase. Bone 27, 575–584 (2000).
Chao, T. Y., Wu, Y. Y. & Janckila, A. J. Tartrate-resistant acid phosphatase isoform 5b (TRACP 5b) as a serum maker for cancer with bone metastasis. Clin. Chim. Acta 411, 1553–1564 (2010).
Burgess, T. L. et al. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J. Cell Biol. 145, 527–538 (1999).
Honig, A. et al. Increased tartrate-resistant acid phosphatase (TRAP) expression in malignant breast, ovarian and melanoma tissue: an investigational study. BMC Cancer 6, 199 (2006).
Zenger, S. et al. Differential expression of tartrate-resistant acid phosphatase isoforms 5a and 5b by tumor and stromal cells in human metastatic bone disease. Clin. Exp. Metastasis 28, 65–73 (2011).
Scott, K. L. et al. Proinvasion metastasis drivers in early-stage melanoma are oncogenes. Cancer Cell 20, 92–103 (2011).
Wu, L. et al. FUN14 domain-containing 1 promotes breast cancer proliferation and migration by activating calcium-NFATC1-BMI1 axis. EBioMedicine 41, 384–394 (2019).
Jiang, Y. et al. Transcription factor NFAT5 contributes to the glycolytic phenotype rewiring and pancreatic cancer progression via transcription of PGK1. Cell Death Dis. 10, 948 (2019).
Acknowledgements
We thank the members of the Zhu lab for their helpful discussion.
Funding
This work was supported by the funds from the Research Start-up Fund of the Seventh Affiliated Hospital, Sun Yat-sen University (ZSQYBRJH0003), and the National Natural Science Foundation of China (82072905 and 81802666).
Author information
Authors and Affiliations
Contributions
C.M.Z. and L.P.Y. conceived and designed the project, analyzed data, and critically revised the manuscript. Q.L. and Y.W. designed and performed experiments, analyzed data, wrote, and edited the manuscript. Y.S.L., Q.Q.Z., W.L.X., N.N.T., L.F.H., T.L.A., D.Z., and A.Q.Y. performed experiments, analyzed data, and critically revised the manuscript. S.Y.L. designed experiments, provided instructions, and critically revised the manuscript. All the authors read and approved the final paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics statement
This study was approved by the Ethics Committee of Sun Yat-sen University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by S. Tait
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liang, Q., Wang, Y., Lu, Y. et al. RANK promotes colorectal cancer migration and invasion by activating the Ca2+-calcineurin/NFATC1-ACP5 axis. Cell Death Dis 12, 336 (2021). https://doi.org/10.1038/s41419-021-03642-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-021-03642-7
This article is cited by
-
Downregulation of osteoprotegerin in colorectal cancer cells promotes liver metastasis via activating tumor-associated macrophage
Scientific Reports (2023)
-
USP7-mediated JUND suppresses RCAN2 transcription and elevates NFATC1 to enhance stem cell property in colorectal cancer
Cell Biology and Toxicology (2023)
-
Immunohistochemical analysis of the distribution of RANKL: a case of disseminated carcinomatosis of bone marrow as the first presentation of relapse in curatively resected colorectal cancer
Medical Molecular Morphology (2023)