Abstract
We investigated the mechanism underlying the effect of a combination treatment of 125I radioactive seed implantation and lobaplatin (LBP) in hepatocellular carcinoma. The effects of administration of HCC cells and subcutaneous tumor model of mice with different doses of 125I or a sensitizing concentration of LBP alone, or in combination, on cellular apoptosis and proliferation were analyzed and it was confirmed that LBP promotes 125I-induced apoptosis and inhibition of proliferation of HCC. Furthermore, isobaric tag for relative and absolute quantification labeling analyses suggested that 125I promoted the apoptosis and inhibition of proliferation of HCC cells by upregulating the expression of PERK-eIF2α-ATF4-CHOP pathway, a well-known apoptosis-related pathway. Moreover, LBP was found to boost the 125I-induced upregulation of this pathway and increase the apoptosis. Our data indicate that LBP promotes the apoptotic and anti-proliferative effects of 125I and provide a firm foundation for better clinical application of this combination therapy.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the third most common malignant cancer in China and has a serious negative effect on patient’s health. More than three million people die from HCC every year in China, especially in rural areas1. For inoperable HCC patients, radiotherapy (RT) alone does not improve the overall survival. Recently, 125I seed implantation has been proven to be a safe, efficacious, and economical method for treating moderate and advanced HCC. However, RT when combined with other treatments, such as platinum chemotherapy, exhibits a better prognosis than the non-RT therapies2,3. Due to the mechanisms underlying the effects of 125I seed in HCC and enhancement of the radiosensitivity of HCC to 125I seed by chemotherapy are unclear, identification of new cellular targets of 125I seed would lay a solid foundation for better clinical application of 125I seed implantation therapy and would provide novel therapeutic approaches for treating HCC.
Endoplasmic reticulum (ER) is an important organelle in cells. Damage of its function causes stress reaction in ER, which is known as ER stress. ER protects cells from the damage caused by such stress, by activating the unfolded protein response (UPR)4,5. The UPR relies on the duration of exposure of cells to unfavorable conditions, such as radiation, which may have disparate outcomes, such as adaptation to the stress or apoptosis6. A proper UPR aims to reduce the ER capacity and protein synthesis, causing the cells to adapt to the stress. However, in the event of an insufficient adaptive response, ER stress induces cells to go through apoptosis and regulates C/EBP homologous protein (CHOP), JNK activation, and Bcl-2 expression7. The PERK-eIF2α-ATF4-CHOP pathway plays an important role in ER stress; it induces apoptosis through upregulation of CHOP, Bcl-2, and other apoptosis-related factors.
As a third-generation platinum drug, lobaplatin (LBP) is reported to induce apoptosis and cell cycle arrest, and impairs the migration and invasion in various gastrointestinal tumor cell lines in vitro8,9. Cells at the G2/M transition stage are more sensitive to RT, indicating that LBP might enhance the radiosensitivity of HCC and ultimately decrease the biologically effective dose, serving to reduce RT-related complications10,11. A retrospective study showed that transarterial chemoembolization (TACE) with gelatin sponge microparticles mixed with LBP is a safe and effective method for stage B HCC patients12. Moreover, Peng et al.13 reported that the combination of LBP-TACE and brachytherapy has a better overall survival than that of LBP-TACE alone; thus, a comprehensive therapy is recommended for these patients13.
Based on the results of isobaric tag for relative and absolute quantification labeling (iTRAQ) and the function of PERK-eIF2α-ATF4-CHOP pathway, we hypothesized that 125I seeds might induce the upregulation of PERK-eIF2a-ATF4-CHOP pathway, resulting in apoptosis in liver cancer cells. Moreover, we verified that LBP could enhance the apoptosis and anti-proliferative activity of 125I, and assumed that this enhancement might work by regulating the PERK-eIF2α-ATF4-CHOP pathway. To test these hypotheses, the correlation between 125I and PERK-eIF2α-ATF4-CHOP pathway was evaluated in liver cancer cell lines and mice tumor model. We found that the PERK-eIF2α-ATF4-CHOP pathway was inhibited in liver cancer cells after treatment with 125I and LBP. Our results indicate that 125I induces the upregulation of PERK-eIF2a-ATF4-CHOP pathway to promote apoptosis and LBP promotes 125I-induced apoptosis by increasing the 125I-induced upregulation of PERK-eIF2α-ATF4-CHOP pathway. In summary, our data identify PERK-eIF2a-ATF4-CHOP pathway as a new mechanism of apoptosis induced by 125I and suggest that PERK-eIF2a-ATF4-CHOP pathway could be a new therapeutic target in 125I seed implantation therapy for HCC.
Materials and methods
Mice subcutaneous tumor formation assay
For xenograft tumor studies, 100 μl of SMMC7721 cells (1 × 107/ml) transfected with PERK-RNAi or Control-RNAi were diluted in 0.9% saline solution and injected subcutaneously in the hind leg of BALB/c male mice (purchased from the Animal Research Center of Shandong University). When the volume of tumor reached 500 mm3, the mice were randomly divided into three group with four mice in each group. Tumor diameters and weight were measured every other day for 30 days, at which time mice were killed and tumors were excised, measured, and lysed for RNA isolation. All mice were housed under specific pathogen-free conditions and were killed according to regulations formulated by the Shandong University Animal Care Committee.
Procedure for radioactive 125I seeds implantation in mice
First of all, the skin on the surface of the tumor was sterilized with iodine. After local anesthesia with lidocaine, the subcutaneous tumor was punctured with an 18G needle (Kakko, Japan) under direct vision. The endpoint of the needle was located at the middle of the tumor. The seed was advanced into the tumor through the needle using seed implantation equipment (Ningbo Junan Pharmaceutical Technology Company, Nanjing, China). When the procedure was completed, a sterile gauze was pressed to stop the bleeds, if necessary.
Radiation for HCC cell lines
125I seeds were purchased from Ningbo Junan Pharmaceutical Technology Company (Ningbo, Zhejiang, China). 125I seeds were seeded into an in vitro irradiation model, based on a previous method14. The initial activity and dose rate were 3.0 mCi and 3.412 cGy/h, respectively. To deliver doses of 1, 2, and 4 Gy, irradiation times of 29.3, 58.6, and 117.2 h, respectively, were used.
Cell lines and transfection
The HCC cell lines were purchased from the Chinese Academy of Sciences (Shanghai, China). The cells were incubated in 5% CO2 at 37 °C and grown in culture media supplemented with 10% fetal calf serum (Gibco, Waltham, MA USA). Human HCC cell line HepG2 was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Corning, Inc., Corning, NY, USA). Human HCC cell line SMMC7721 was cultured in RPMI 1640 (Corning, Inc., Corning, NY, USA).
Transfections were performed using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s instructions. A short interfering RNA (siRNA) for PERK (protein kinase RNA-like ER kinase) was designed by RiboBio (Guangzhou, Guangdong, China). PERK-RNAi was purchased from Genechem (Shanghai, China).
iTRAQ labeling
HepG2 cells were treated with or without 125I and total protein was isolated with RIPA (TIANGEN). The iTRAQ was performed by Jiyun Biotech (Shanghai, China) in 125I-treated HepG2 cells and control HepG2 cells. The signal pathway enrichment was analyzed using Ingenuity Pathway Analysis (INGENUITY). The mean value between the 125I-treated group and control group, which is >1.2 and p < 0.05, is supposed to be significantly upregulated.
Selection for sensitizing concentration of LBP to HCC cells
HCC cells were seeded in a 96-well plate. After treatment with concentrations of LBP ranging from 0 to 80 µg/mL for 72 h, the optical density was detected using CCK-8 assays (Dojindo, Kumamoto, Japan). The half-maximal inhibitory concentration (IC50) was calculated with Prism 6.0 (GraphPad, San Diego, CA, USA). According to the toxic effects of LBP on HCC cells and previously published method, the LBP-sensitizing concentration was determined to be 10% of the IC5015.
Cell proliferation assay
After treatment, cells were collected as a single cell suspension and were seeded in 96-well plates at 5 × 103 cells/well, with each well containing 200 µL culture medium. The CCK-8 reagent was added into the medium at 24, 48, and 72 h after the cell was attached and then incubated at 37 °C for 3 h. Finally, the plate was scanned by a microplate reader (Thermo, Waltham, MA, USA).
Cell cycle and apoptosis analysis
Cells were seeded into six-well plates at 2 × 105 cells per well and were treated as indicated. For the cell cycle assay, after collection, the cells were washed with cold phosphate-buffered saline (PBS), fixed with 70% cold ethanol, and stored at −4 °C overnight. Before analysis, the cells were stained with propidium iodide (PI) and RNase A (BD Biosciences, Franklin Lakes, NJ, USA). For the apoptosis analysis, after collecting, cells were either stained with PI and Annexin V–FITC (BD Biosciences) or Annexin V–APC (Elabscience, Wuhan, China). Both the cell cycle and apoptosis assays were assessed using a flow cytometer (BD Biosciences).
Surviving fractions
After being treated as indicated, the cells were collected as a single cell suspension and were seeded in six-well plates at 103 cells/well, with each well containing 3 mL culture medium. After incubation on the plates for 10–14 days, the colonies were washed once with PBS, fixed with 4% paraformaldehyde for at least 1 h, and were stained with crystal violet for 30 min. Colonies containing more than 50 cells were counted in 3 replicate dishes. The surviving fraction (SF) was defined as the ratio of PE (125I + LBP)/PE (125I), with PE defined as (colony number/number of seeded cells) × 100.
Terminal deoxynucleotidyl transferase dUTP nick end labeling assay
After treatment as indicated, the cells were washed once with 1 × PBS and treated with PBS containing 0.3% Triton X-100 for 5 min. Then, the cells were fixed in 4% paraformaldehyde for at least 1 h, stained with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) reagent (Beyotime Biotechnology, Nanjing, Jiangsu, China) for 1 h at 37 °C, and incubated with 4′,6-diamidino-2-phenylindole for another 15 min. Morphological changes were observed using a fluorescence microscope (Olympus, Tokyo, Japan). Three different fields were selected. Cells were counted using Image J.
EdU staining assay
The cell proliferation was detected using Cell-Light EdU Apollo567 In Vitro Kit (RiboBio, Guangdong, China) according to the manufacturer’s instructions. After treatment, the cell medium was replaced with 50 μM EdU solution diluted with DMEM and incubated for 2 h. Hoechst 33342 was used to stain the nuclei and cell counting was done using a fluorescence microscope (Olympus, Tokyo, Japan). Cells were counted using Image J.
Western blotting and antibodies
After treatment as indicated, the cells were washed with cold 1 × PBS, lysed with RIPA lysis buffer for 20 min on ice, and centrifuged at 12,000 r.p.m. for 10 min at 4 °C. Protein was denatured at 95 °C for 5 min. Samples (20 µg each) were analyzed by 10–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Millipore, Burlington, MA, USA). Membranes were blocked with 5% nonfat milk in Tris-buffered saline containing 1% Tween-20 for 1 h and incubated with corresponding primary antibodies at 4 °C overnight. Then, the membranes were washed with Tris-buffered saline containing Tween-20 three times for 5 min each and incubated with corresponding secondary antibodies for 1 h at room temperature. Protein brands were detected by chemiluminescence (Millipore).
The primary antibodies anti-Bax (ab32503), anti-Bcl-2 (ab692), anti-ATF4 (ab184909), anti-CHOP (ab11419), anti-eIF2A (ab169528), anti-eIF2S (ab32157), and anti-PERK (ab79483) were purchased from Abcam (Cambridge, UK). Anti-phospho-PERK was purchased from Cell Signaling Technology (Beverly, MA, USA). The following secondary antibodies were purchased from GenScript (Piscataway, NJ, USA): goat anti-rabbit IgG (H&L), goat anti-mouse IgG (H&L), and rabbit anti-goat IgG. LBP was purchased from Changan Hainan International Pharmaceutical Co., Ltd (Haikou, Hainan, China).
RNA isolation and qPCR
Expression levels of PERK, eIF2a (eukaryotic initiation factor 2α), ATF4 (activating transcription factor 4), and CHOP were determined using quantitative real-time PCR. Total RNA was isolated from tissues and cultured cells using TRIzol reagent (TIANGEN, Beijing, China) according to the manufacturer’s instructions. cDNA was synthesized from 1.0 μg RNA using a FastQuant RT Kit (TIANGEN). Quantitative PCR (qPCR) was performed by using BioRad C1000 Thermal Cycler CFX96 Real-Time System with SuperReal PreMix Plus (SYBR Green, TaKaRa). mRNA expression were normalized using detection of β-actin, respectively. Primers for qPCR were as follows: PERK, forward primer: 5′-ACGATGAGACAGAGTTGCGAC-3′, reverse primer: 5′-ATCCAAGGCAGCAATTCTCCC-3′; eIF2a, forward primer: 5′-CCGCTCTTGACAGTCCGAG-3′, reverse primer: 5′-GCAGTAGTCCCTTGTTAGTGACA-3′; ATF4, forward primer: 5′-ATGACCGAAATGAGCTTCCTG-3′, reverse primer: 5′-GCTGGAGAACCCATGAGGT-3′; CHOP, forward primer: 5′-GGAAACAGAGTGGTCATTCCC-3′, reverse primer: 5′-CTGCTTGAGCCGTTCATTCTC-3′; β-actin, forward primer: 5′-TCCCTGGAGAAGAGCTACCA-3′, reverse primer: 5′-AGCACTGTGTTGGCGTACAG-3′.
Statistical analysis
Data are reported as the mean ± SD. All experiments were repeated at least twice. GraphPad Prism software version 6.0 was used for statistical analysis. One-way analysis of variance with Tukey’s multiple comparison test was utilized to analyze the subcutaneous tumor growth. An unpaired t-test was used when appropriate. *P < 0.05, **P < 0.01, and ***P < 0.001 were assumed.
Results
LBP increases 125I-induced apoptosis in HCC cells
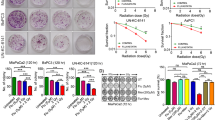
To investigate the radiosensitization effect of LBP on HCC cells to 125I, the sensitization concentration of LBP was determined in HepG2 and SMMC7721 cell lines. The sensitization concentration was defined as 10% of the IC50. The CCK-8 assay suggested that LBP affected the viability of HepG2 and SMMC7721 cells in a dose-dependent manner. The estimated IC50 value of LBP at 117.2 h on HepG2 and SMMC7721 cells was found to be 5.972 µg/mL and 1.536 µg/mL, respectively (Fig. 1a).
a To investigate the radiosensitization effect of LBP on HCC cells to 125I, the concentration of LBP required for sensitization of HepG2 and SMMC7721 cell lines was determined. After treatment with LBP concentrations ranging from 0 to 80 µg/mL for 72 h, the optical density value was detected using a CCK-8 assay. The sensitization concentration was defined as 10% of IC50. b Cells were treated with 125I or 125I + LBP. After treatment, cells were plated in fresh medium for 10–14 days to assess cell survival with a colony formation assay. A single-hit multitarget model was used to construct dose-survival curves and to estimate sensitizer enhancement ratios. Based on our results, the sensitizer enhancement ratios for HepG2 and SMMC7721 cells were 1.37 and 1.63, respectively. c, d After cells were treated with 125I, LBP, or 125I + LBP, Annexin V–FITC/PI and TUNEL assays were performed to analyze cellular apoptosis. All experiments were performed in triplicate and the data are presented as the mean ± SD. The t-test was used for data analysis. *P < 0.05, **P < 0.01
To estimate the radiosensitizing effect of LBP, HepG2 and SMMC7721 cells were exposed to 125I, with and without concurrent treatment with LBP (Fig. 1b). The SF of HepG2 cells treated with 125I alone at 4 Gy was 0.071 ± 0.009, and that with 125I and LBP was 0.033 ± 0.005. The SF of SMMC7721 cells treated with 125I alone and with a combination of 4 Gy 125I and LBP was 0.061 ± 0.015 and 0.011 ± 0.005, respectively. The sensitizer enhancement ratios for HepG2 and SMMC7721 cells were 1.37 and 1.63, respectively. The radio-biological parameters of HCC cells are shown in Table 1.
To investigate the promotion of 125I-induced effects on cellular apoptosis by LBP, Annexin V–FITC/PI assay (Fig. 1c) and TUNEL assay (Fig. 1d) were performed in both HepG2 and SMMC7721 cells subjected to single or combined treatments. The Annexin V–FITC/PI assay revealed that when the HCC cells were treated with a combination of 125I and LBP, cellular apoptosis was significantly higher than that observed when the cells were treated with 125I or LBP alone (Fig. 1c). Furthermore, TUNEL assay results showed that LBP increased the 125I-induced cellular apoptosis in both HepG2 and SMMC7721 cells (Fig. 1d). The apoptosis was increased in the combination treatment group compared with that in the treatments with a single agent. Interestingly, the SMMC7721 cells showed more positive results than HepG2, which was consistent with the results of the radiosensitizing effect.
LBP promotes 125I-induced anti-proliferative effect in HCC cells
To investigate whether the 125I-induced anti-proliferative effect on HCC cells was promoted by LBP, a cell proliferation assay, cell cycle assay, and EdU assay were performed for both HepG2 and SMMC7721 cells treated with 125I, LBP, or a combination of the two (Fig. 2). Results of the cell proliferation assay suggested that the growth of HepG2 and SMMC7721 cells was inhibited following treatment with 125I or LBP alone. However, the inhibition of cell growth was more significant in the combined treatment group (Fig. 2a). Furthermore, the results of cell cycle analysis showed that both 125I and LBP induced cell cycle arrest at the G2/M transition. In addition, the combined treatment resulted in more obvious cell cycle arrest (Fig. 2b). Besides, results of the EdU assay showed that the combination treatment clearly exhibited fewer cells with proliferative status (Fig. 2c).
a To investigate whether the 125I-induced anti-proliferative effect in HCC cells was promoted by LBP, proliferation of both HepG2 and SMMC7721 cells treated with 125I, LBP, or a combination of the two was detected by CCK-8 assay. b, c Cell cycle and EdU assay were performed to verify the anti-proliferation effect of LBP and 125I on HepG2 and SMMC7721 cells. According to our results, the inhibition of cell growth was more significant in the combined treatment group than in the single treatment group. All experiments were performed in triplicate and the data are presented as the mean ± SD. The t-test was used for data analysis. *P < 0.05, **P < 0.01
125I induces upregulation of the PERK-eIF2a-ATF4-CHOP pathway to promote apoptosis
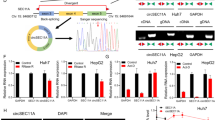
We hypothesized that 125I radioactive seeds induce apoptosis and inhibit proliferation of HCC cells via multiple pathways, including regulation of apoptosis-related proteins (Fig. 3). To test this hypothesis, iTRAQ was performed in control and 125I-treated HepG2 cells. Among the differentially expressed proteins, we selected those involved in the eIF2 signaling pathway, which was the most enriched pathway. Furthermore, we identified the PERK-eIF2α-ATF4-CHOP pathway, a well-known ER stress pathway, to be potentially involved in 125I-induced apoptosis (Fig. 3a).
a To explore the possible apoptosis-related pathways induced by 125I, iTRAQ was performed in HepG2 cells. The results of the signal pathway enrichment analysis of differentially expressed proteins reveals that the eIF2 signaling pathway is significantly different between the control HepG2 cells and 125I-treated HepG2 cells. b, c After HepG2 cells were treated with different doses of 125I, the proteins and mRNA levels of the PERK-eIF2-ATF4-CHOP pathway were upregulated in a dose-dependent manner, as detected by WB (b) and qPCR (c). d The mRNA levels consistently increased in animal models in a dose-dependent manner, after 125I irradiation. Total RNA was isolated from mouse tumors and the pathway-related genes were seen to be upregulated at the mRNA level, as detected by qPCR. e, f The 125I-induced apoptosis and anti-proliferation effect were compromised by PERK-RNAi. After HepG2 cells transfected with Control-RNAi or PERK-RNAi were treated by 125I, the apoptosis and anti-proliferation effect were detected by cell proliferation assay and Annexin V–FITC/PI assay. The PERK-RNAi abrogated 125I-induced apoptosis and anti-proliferation effect. All the experiments were performed in triplicate and the data are presented as the mean ± SD. The t-test was used for data analysis. *P < 0.05, **P < 0.01
To confirm the relationship between 125I and the PERK-eIF2α-ATF4-CHOP pathway, pathway-related proteins were detected after HepG2 cells were treated with 125I. The results of western blotting and qPCR demonstrated that the expression levels of the selected protein and mRNA were significantly increased in a dose-dependent manner (Fig. 3b, c). Similar results were obtained in the mouse tumor model. The expression of the pathway-related genes was also upregulated at the mRNA level, as detected by qPCR (Fig. 3d). Furthermore, to verify whether 125I induces apoptosis through the PERK-eIF2α-ATF4-CHOP pathway, PERK-RNAi and Control-RNAi were transfected into HepG2 cells. After treatment with 125I, a cell proliferation assay was performed to evaluate the effect of 125I on anti-proliferation (Fig. 3e). According to our results, the PERK-RNAi abrogated the 125I-induced anti-proliferation effect. Apoptosis was detected by the Annexin V–FITC/PI assay. Compared with the cells transfected with Control-RNAi, the apoptosis rate was reduced in PERK-RNAi-transfected cells. Taken together, these data indicate that 125I induces the upregulation of the ER stress pathway to promote apoptosis (Fig. 3f).
LBP increases the 125I-induced upregulation of the PERK-eIF2α-ATF4-CHOP pathway to promote 125I-induced apoptosis
To further confirm the sensitizer role of LBP in 125I-mediated apoptosis and anti-proliferative effect in HCC cells, the PERK-eIF2α-ATF4-CHOP pathway-related proteins were detected after SMMC7721 cells were treated with 125I or a combination of 125I and LBP (Fig. 4a). The results showed that the expression levels of the selected proteins were significantly increased in the combination treatment group compared with their levels in the group that was treated with 125I alone.
a LBP boosts the 125I-induced upregulation of the PERK-eIF2α-ATF4-CHOP pathway. To further confirm the role of LBP as a sensitizer in 125I-mediated apoptosis and anti-proliferative effect in HCC cells, the PERK-eIF2α-ATF4-CHOP pathway-related proteins were detected by WB after the treatment of SMMC7721 cells with 125I or a combination of 125I and LBP. b–d The combination of 125I and LBP induced apoptosis and anti-proliferative effect on HCC cells that could be compromised by PERK-RNAi. After SMMC7721 and HepG2 cells transfected with Control-RNAi or PERK-RNAi were treated with 125I and LBP, the cell cycle (b), Bax/Bcl-2 ratio (c), and Annexin V–APC/PI assay (d) were performed to verify the rescue effect on apoptosis and anti-proliferation. All the experiments were performed in triplicate and the data are presented as the mean ± SD. The t-test was used for data analysis. *P < 0.05, **P < 0.01
To further verify the role of the PERK-eIF2α-ATF4-CHOP pathway in the 125I and LBP coinduced apoptosis and anti-proliferation of HCC cells, cell cycle (B), Bax/Bcl-2 ratio (C), and Annexin V–APC/PI assays (D) were performed. After SMMC7721 and HepG2 cells were transfected with Control-RNAi or PERK-RNAi, cells were treated with 125I and LBP. According to the results, the apoptosis and anti-proliferation induced by the combination of 125I and LBP in HCC cells could be compromised by PERK-RNAi. Compared with the cells transfected with Control-RNAi, the G2/M rate, Bax/Bcl-2 ratio, and apoptosis rate were decreased in cells transfected with PERK-RNAi. Taken together, these results suggested that LBP promotes 125I-induced apoptosis via increasing the 125I-induced upregulation of the PERK-eIF2α-ATF4-CHOP pathway.
PERK downregulation compromises the inhibition of HCC growth induced by 125I and LBP in nude mouse SMMC7721 xenograft tumors
To verify the role of the PERK-eIF2α-ATF4-CHOP pathway in 125I-mediated apoptosis and anti-proliferation, SMMC7721 xenograft tumors were treated with 125I, LBP, and RNAi (PERK-RNAi and Control-RNAi). The results showed that, compared with the 125I treatment alone, the combination treatment inhibited the tumor growth more significantly (Fig. 5). As shown in the Fig. 5a, the combination of 125I and LBP inhibited tumor growth more cooperatively than 125I treatment alone. However, when the cells were transfected with PERK-RNAi, inhibition of tumor growth was reduced compared with that in the cells transfected with Control-RNAi. Consistent with the diminished growth curves, tumor weights at the time of killing were also significantly reduced in mice treated with the combined treatment compared with mice treated with 125I alone (Fig. 5b). Moreover, the tumor volume was more obviously decreased by the combined treatment than by treatment with 125I alone (Fig. 5c). In general, these data indicated that LBP promotes 125I-induced anti-proliferation in liver cancer cells. Overall, these results indicate that LBP increases the 125I-induced upregulation of the PERK-eIF2α-ATF4-CHOP pathway to promote apoptosis and the inhibition of proliferation.
To further verify the effect of the PERK-eIF2α-ATF4-CHOP pathway on 125I-mediated apoptosis and anti-proliferation of HCC cells, SMMC7721 cells transfected with PERK-RNAi or Control-RNAi xenograft tumors were treated with 125I and LBP. When the volume of the tumors reached 500 mm3, the mice were randomly divided to three groups with four mice in each group. After 30 days of treatment, the mice were killed and the tumors were exfoliated. The tumor weight (b) and diameters (c) were measured every other day for 30 days. One-way ANOVA with Tukey’s multiple comparison test was utilized to analyze the subcutaneous tumor growth. All the experiments were performed in triplicate and the data are presented as the mean ± SD. The t-test was used for data analysis. *P < 0.05, **P < 0.01
Discussion
125I seed implantation has recently become a standard treatment for the management of HCC12,16,17,18. The risk of radiological complications is correlated with the dose, treatment volume, and dose rate19. A cardinal principle of 125I seed implantation is to use the minimal dose, also known as the biologically effective dose, at which tumor cells are killed but neighboring normal cells are spared. Decreasing the total dose is a feasible method for preventing radiological complications. In a previous study, we showed that 125I induces apoptosis and inhibits proliferation in pancreatic cancer cells20. However, the mechanism by which 125I induces apoptosis and inhibits proliferation in HCC is still unknown. Based on the results of iTRAQ, eIF2 signaling pathway was found to be the most enriched pathway. We identified the PERK-eIF2a-ATF4-CHOP pathway to potentially participate in 125I-induced apoptosis. Here we investigated whether the radiosensitivity of HCC to 125I could be promoted by LBP and the possible links between 125I, LBP, and PERK-eIF2α-ATF4-CHOP pathway.
LBP has been approved for the treatment of several cancers, including liver, stomach, lung, and nasopharyngeal cancers, and the combination of 125I seeds and LBP results in improved prognosis in the treatment of hepatic cancer21,22,23. A retrospective study showed that TACE with gelatin sponge microparticles mixed with LBP is a safe and effective method for stage B HCC patients12. Futhermore, it was reported that the combination of LBP-TACE and 125I radioactive seeds results in a better overall survival than LBP-TACE therapy alone; thus, a comprehensive therapy is recommended for these patients13. The present study indicates that LBP causes significant apoptosis and inhibition of proliferation in HCC cells, similar to that observed in 125I seed RT alone, whereas a combination of the two treatments results in an even greater effect in vitro and in vivo. These results indicate that LBP promotes 125I-induced apoptosis and inhibition of proliferation in HCC.
A possible pathway involved in the 125I-mediated apoptosis and inhibition of proliferation in HCC cells could be the PERK-eIF2α-ATF4-CHOP pathway. The activated PERK elicits UPR-related pro-apoptotic signals, markedly elevating the levels of phosphorylated eIF2α. This results in the activation of a pro-adaptive signaling pathway via the inhibition of global protein synthesis and selective translation of ATF4, a transcription factor that regulates many genes related to the recovery of cells and their adaption to stress24. However, ATF4 was found to be related to 5-fluorouracil resistance in colorectal cancer25. Interestingly, the ATF4 levels were upregulated after treatment with gemcitabine in pancreatic cancer cells and could be regarded as a mechanism for induction of apoptosis26. Furthermore, ATF4 increases the expression levels of transcription factor CHOP. Overexpression of CHOP downregulates the expression of Bcl-2, upregulates Bax, and evokes apoptosis7. In addition, CHOP plays an important role in another pro-apoptotic mechanism, because it directly activates the growth arrest and DNA damage-inducible protein, GADD34, which sufficiently promotes the dephosphorylation of eIF2α and reboots protein translation in stressed cells27. Our results showed that 125I upregulated the PERK-eIF2α-ATF4-CHOP pathway in a dose-dependent manner. Besides, the apoptosis induced by 125I was reduced when PERK was downregulated by PERK-siRNA. More importantly, not only did LBP enhance the upregulation of the PERK pathway induced by 125I, the inhibition of tumor growth in mouse tumor model treated with combined treatment was compromised when PERK-siRNA was transfected into HCC cells, which might explain the mechanism by which LBP promotes the radiosensitivity of HCC cells to 125I.
Our results also show that LBP significantly increased the 125I-induced cell cycle arrest in HepG2 cells. The ratio of cells in the G2/M stage in the combination treatment group was markedly higher than that in the 125I seed radiation group. Univariate general linear model analysis showed that the arrest of HCC cells was greater in the combination treatment group than in the groups treated with either of the agents alone, indicating that LBP might have a synergistic effect with 125I seeds. Dai et al.8 reported that cell cycle arrest might be associated with the downregulation of CDK1, phosphorylated CDK1 (p-CDK1), cyclin B, CDC25C, p-CDK4, Rb, p-Rb, and E2F, and the upregulation of p27, p21, and p538. Besides, cells at G2/M are more sensitive to radiation, which might explain the ability of LBP to promote radiosensitivity of HCC cells to 125I seeds.
The data in this study indicate that LBP promotes the radiosensitivity of HCC cells to radioactive 125I seeds. In addition, when comparing the radiosensitivity of HepG2 and SMMC7721 cells with 125I seeds, it is interesting to find that the latter are more sensitive to 125I seeds. The sensitization enhancement ratio for HepG2 and SMMC7721 was 1.37 and 1.63, respectively, indicating that a lower dose could be used to kill hepatic cancer cells when 125I seed radiation is combined with concurrent LBP chemotherapy. We believe that the different effect between the two cell lines could be due to the different expression levels of ZHX2. ZHX2 has been reported to function as a tumor suppressor in the development of HCC and the reduced ZHX2 level also leads to a lower response to chemotherapeutic drugs28,29,30. Thus, further study is needed to investigate the link between 125I seeds, radiosensitivity, and ZXH2 ZHX2.
Conclusion
In summary, the data in this study reveal a relationship between 125I, LBP, and the PERK-eIF2α-ATF4-CHOP pathway. LBP promotes 125I-induced apoptosis and inhibition of proliferation of HCC by upregulating the PERK-eIF2α-ATF4-CHOP pathway. Furthermore, our data lays a solid foundation for better clinical application of this combined therapy.
Data availability
All authors declare that all data and materials described in the manuscript will be freely available to any scientist wishing to use them for non-commercial purposes.
References
Chen, W. et al. Cancer statistics in China, 2015. Cancer J. Clin. 66, 115–132 (2016).
Liu, S. M. et al. The efficacy of iodine-125 permanent brachytherapy versus intensity-modulated radiation for inoperable salivary gland malignancies: study protocol of a randomised controlled trial. BMC Cancer 16, 193 (2016).
Meng, M. et al. Stereotactic body radiation therapy: a novel treatment modality for inoperable hepatocellular carcinoma. Drug Discov. Ther. 9, 372–379 (2015).
Yao, Y. et al. A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat. Commun. 8, 133 (2017).
Park, H. W. et al. Hepatoprotective role of Sestrin2 against chronic ER stress. Nat. Commun. 5, 4233 (2014).
Vandewynckel, Y. P. et al. The paradox of the unfolded protein response in cancer. Anticancer Res. 33, 4683–4694 (2013).
Su, J. et al. Bcl-2 family proteins are involved in the signal crosstalk between endoplasmic reticulum stress and mitochondrial dysfunction in tumor chemotherapy resistance. BioMed. Res. Int. 2014, 234370 (2014).
Dai, H. Y., Liu, L., Qin, S. K., He, X. M. & Li, S. Y. Lobaplatin suppresses proliferation and induces apoptosis in the human colorectal carcinoma cell Line LOVO in vitro. Biomedi. Pharmacother. 65, 137–141 (2011).
Li, Y. et al. Lobaplatin induces BGC-823 human gastric carcinoma cell apoptosis via ROS- mitochondrial apoptotic pathway and impairs cell migration and invasion. Biomed. Pharmacother. 83, 1239–1246 (2016).
Wu, Q., Qin, S. K., Teng, F. M., Chen, C. J. & Wang, R. Lobaplatin arrests cell cycle progression in human hepatocellular carcinoma cells. J. Hematol. Oncol. 3, 43 (2010).
Wang, Z. et al. Lobaplatin induces apoptosis and arrests cell cycle progression in human cholangiocarcinoma cell line RBE. Biomed. Pharmacother. 66, 161–166 (2012).
Kamran, A. U. et al. Transcatheter arterial chemoembolization with gelatin sponge microparticles treated for BCLC stage B hepatocellular carcinoma: a single center retrospective study. Medicine 94, e2154 (2015).
Peng, S. et al. Lobaplatin-TACE combined with radioactive 125I seed implantation for treatment of primary hepatocellular carcinoma. Asian Pac. J. cancer Prev. 15, 5155–5160 (2014).
Liu, J. et al. Combined effects of C225 and 125-iodine seed radiation on colorectal cancer cells. Radiat. Oncol. 8, 219 (2013).
Kanzawa, F. et al. In vitro synergistic interactions between the cisplatin analogue nedaplatin and the DNA topoisomerase I inhibitor irinotecan and the mechanism of this interaction. Clin. Cancer Res. 7, 202–209 (2001).
Yang, Q. et al. Spectral CT with monochromatic imaging and metal artifacts reduction software for artifacts reduction of (1)(2)(5)I radioactive seeds in liver brachytherapy. Jpn J. Radiol. 33, 694–705 (2015).
Sun, J. H. et al. Portal vein stenting combined with iodine-125 seeds endovascular implantation followed by transcatheter arterial chemoembolization for treatment of hepatocellular carcinoma patients with portal vein tumor thrombus. BioMed. Res. Int. 2016, 3048261 (2016).
Liu, Y., Liu, R., Wang, P., Li, S. & Shen, H. Percutaneous implantation of (125)iodine seeds for treatment of portal vein tumor thrombosis in hepatocellular carcinoma. Med. Oncol. 32, 214 (2015).
Escande, A. et al. Brachytherapy for conservative treatment of invasive penile carcinoma: prognostic factors and long-term analysis of outcome. Int. J. Radiat. Oncol. Biol. Phys. 99, 563–570 (2017).
Li, D. et al. Combined effect of (125)I and gemcitabine on PANC-1 cells: cellular apoptosis and cell cycle arrest. J. Cancer Res. Ther. 14, 1476–1481 (2018).
Yin, C. Y. et al. Lobaplatin inhibits growth of gastric cancer cells by inducing apoptosis. World J. Gastroenterol. 20, 17426–17433 (2014).
Zhang, H. et al. Lobaplatin for the treatment of SK-MES-1 lung squamous cell line in vitro and in vivo. OncoTargets Ther. 9, 4215–4224 (2016).
Zhang, S., Lin, S. & Hu, L. Lobaplatin combined with docetaxel neoadjuvant chemotherapy followed by concurrent lobaplatin with intensity-modulated radiotherapy increases the survival of patients with high-risk lymph node positive nasopharyngeal carcinoma. J. BUON 21, 161–167 (2016).
Khan, I., Tang, E. & Arany, P. Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress. Sci. Rep. 5, 10581 (2015).
Hu, Y. L. et al. Glucose deprivation induces chemoresistance in colorectal cancer cells by increasing ATF4 expression. World J. Gastroenterol. 22, 6235–6245 (2016).
Palam, L. R., Gore, J., Craven, K. E., Wilson, J. L. & Korc, M. Integrated stress response is critical for gemcitabine resistance in pancreatic ductal adenocarcinoma. Cell Death Dis. 6, e1913 (2015).
Rozpedek, W. et al. The role of the PERK/eIF2alpha/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr. Mol. Med. 16, 533–544 (2016).
Song, X. et al. HBV suppresses ZHX2 expression to promote proliferation of HCC through miR-155 activation. Int. J. Cancer 143, 3120–3130 (2018).
Ma, H. et al. ZHX2 enhances the cytotoxicity of chemotherapeutic drugs in liver tumor cells by repressing MDR1 via interfering with NF-YA. Oncotarget 6, 1049–1063 (2015).
Shen, H. et al. ZHX2 is a repressor of alpha-fetoprotein expression in human hepatoma cell lines. J. Cell. Mol. Med. 12, 2772–2780 (2008).
Acknowledgements
This study is supported by the National Natural Science Foundation of China (Grant number 61671276) and the Natural Science Foundation of Shandong Province (Grant number ZR2018PH033).
Author information
Authors and Affiliations
Contributions
D.L.: methodology, project administration, writing of original draft. W.-j.W.: supervision, investigation, data curation. Y.-z.W.: resources and investigation. Y.-b.W.: supervision, project administration, investigation, writing review, and editing. Y.-l.L.: funding acquisition, writing review and editing, supervision, project administration, investigation.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by A. Stephanou
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, D., Wang, Wj., Wang, Yz. et al. Lobaplatin promotes 125I-induced apoptosis and inhibition of proliferation in hepatocellular carcinoma by upregulating PERK-eIF2α-ATF4-CHOP pathway. Cell Death Dis 10, 744 (2019). https://doi.org/10.1038/s41419-019-1918-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-019-1918-1
This article is cited by
-
Characterization of circSEC11A as a novel regulator of Iodine-125 radioactive seed-induced anticancer effects in hepatocellular carcinoma via targeting ZHX2/GADD34 axis
Cell Death Discovery (2023)
-
Differential proteomic analysis of plasma-derived exosomes as diagnostic biomarkers for chronic HBV-related liver disease
Scientific Reports (2022)
-
Activation of the EGFR-PI3K-CaM pathway by PRL-1-overexpressing placenta-derived mesenchymal stem cells ameliorates liver cirrhosis via ER stress-dependent calcium
Stem Cell Research & Therapy (2021)