Abstract
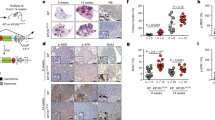
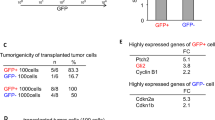
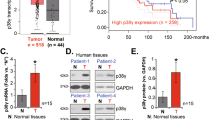
Alterations of the retinoblastoma and/or the p53 signaling network are associated with specific cancers such as high-grade astrocytoma/glioblastoma, small-cell lung cancer (SCLC), choroid plexus tumors, and small-cell pancreatic neuroendocrine carcinoma (SC-PaNEC). However, the intricate functional redundancy between RB1 and the related pocket proteins RBL1/p107 and RBL2/p130 in suppressing tumorigenesis remains poorly understood. Here we performed lineage-restricted parallel inactivation of rb1 and rbl1 by multiplex CRISPR/Cas9 genome editing in the true diploid Xenopus tropicalis to gain insight into this in vivo redundancy. We show that while rb1 inactivation is sufficient to induce choroid plexus papilloma, combined rb1 and rbl1 inactivation is required and sufficient to drive SC-PaNEC, retinoblastoma and astrocytoma. Further, using a novel Li-Fraumeni syndrome-mimicking tp53 mutant X. tropicalis line, we demonstrate increased malignancy of rb1/rbl1-mutant glioma towards glioblastoma upon concomitant inactivation of tp53. Interestingly, although clinical SC-PaNEC samples are characterized by abnormal p53 expression or localization, in the current experimental models, the tp53 status had little effect on the establishment and growth of SC-PaNEC, but may rather be essential for maintaining chromosomal stability. SCLC was only rarely observed in our experimental setup, indicating requirement of additional or alternative oncogenic insults. In conclusion, we used CRISPR/Cas9 to delineate the tumor suppressor properties of Rbl1, generating new insights in the functional redundancy within the retinoblastoma protein family in suppressing neuroendocrine pancreatic cancer and glioma/glioblastoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tong Y, Merino D, Nimmervoll B, Gupta K, Wang Y-D, Finkelstein D, et al. Cross-species genomics identifies TAF12, NFYC, and RAD54L as choroid plexus carcinoma oncogenes. Cancer Cell. 2015;27:712–27.
McLendon R, Friedman A, Bigner D, Van Meir EG, Brat DJ, Mastrogianakis GM, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8.
Chow LML, Endersby R, Zhu X, Rankin S, Qu C, Zhang J, et al. Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell. 2011;19:305–16.
Konukiewitz B, Schlitter AM, Jesinghaus M, Pfister D, Steiger K, Segler A, et al. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20%. Mod Pathol. 2017;30:587–98.
George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524:47–53.
Hann CL, Rudin CM. Management of small-cell lung cancer: incremental changes but hope for the future. Oncology. 2008;22:1486–92.
Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39:683–90.
Tabori U, Shlien A, Baskin B, Levitt S, Ray P, Alon N, et al. TP53 alterations determine clinical subgroups and survival of patients with choroid plexus tumors. J Clin Oncol. 2010;28:1995–2001.
Chow RD, Guzman CD, Wang G, Schmidt F, Youngblood MW, Ye L, et al. AAV-mediated direct in vivo CRISPR screen identifies functional suppressors in glioblastoma. Nat Neurosci. 2017;20:1329–41.
Dannenberg J-H, Schuijff L, Dekker M, Van Der Valk M, Te Riele H. Tissue-specific tumor suppressor activity of retinoblastoma gene homologs p107 and p130. 2004. https://doi.org/10.1101/gad.322004.
Costa C, Paramio JM, Santos M. Skin tumors Rb(eing) uncovered. Front Oncol. 2013;3:307.
Robanus-Maandag E, Dekker M, van der Valk M, Carrozza ML, Jeanny JC, Dannenberg JH, et al. p107 is a suppressor of retinoblastoma development in pRb-deficient mice. Genes Dev. 1998;12:1599–609.
Naert T, Colpaert R, Van Nieuwenhuysen T, Dimitrakopoulou D, Leoen J, Haustraete J, et al. CRISPR/Cas9 mediated knockout of rb1 and rbl1 leads to rapid and penetrant retinoblastoma development in Xenopus tropicalis. Sci Rep. 2016;6. https://doi.org/10.1038/srep35264.
Xiao A, Wu H, Pandolfi PP, Louis DN, Van Dyke T. Astrocyte inactivation of the pRb pathway predisposes mice to malignant astrocytoma development that is accelerated by PTEN mutation. Cancer Cell. 2002;1:157–68.
Lu X, Magrane G, Yin C, Louis DN, Gray J, Van Dyke T. Selective inactivation of p53 facilitates mouse epithelial tumor progression without chromosomal instability. Mol Cell Biol. 2001;21:6017–30.
Naert T, Van Nieuwenhuysen T, Vleminckx K. TALENs and CRISPR/Cas9 fuel genetically engineered clinically relevant Xenopus tropicalis tumor models. Genesis. 2017;55:e23005.
Bougeard G, Renaux-Petel M, Flaman J-M, Charbonnier C, Fermey P, Belotti M, et al. Revisiting Li-fraumeni syndrome from TP53 mutation carriers. J Clin Oncol. 2015;33:2345–52.
Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–21.
Ignatius MS, Hayes MN, Moore FE, Tang Q, Garcia SP, Blackburn PR, et al. tp53 deficiency causes a wide tumor spectrum and increases embryonal rhabdomyosarcoma metastasis in zebrafish. Elife. 2018;7. https://doi.org/10.7554/eLife.37202.
Berghmans S, Murphey RD, Wienholds E, Neuberg D, Kutok JL, Fletcher CDM, et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci. 2005;102:407–12.
Manning AL, Benes C, Dyson NJ. Whole chromosome instability resulting from the synergistic effects of pRB and p53 inactivation. Oncogene. 2014;33:2487–94.
Eischen CM. Genome stability requires p53. Cold Spring Harb Perspect Med. 2016;6:a026096.
Naert T, Vleminckx K. CRISPR/Cas9 disease models in zebrafish and Xenopus: the genetic renaissance of fish and frogs. Drug Discov Today Technol. 2018;28:41–52.
Boel A, Steyaert W, De Rocker N, Menten B, Callewaert B, De Paepe A, et al. BATCH-GE: batch analysis of next-generation sequencing data for genome editing assessment. Sci Rep. 2016;6:30330.
Neely HR, Guo J, Flowers EM, Criscitiello MF, Flajnik MF. ‘Double-duty’ conventional dendritic cells in the amphibian Xenopus as the prototype for antigen presentation to B cells. Eur J Immunol. 2018;48:430–40.
Ward JM, Tadesse-Heath L, Perkins SN, Chattopadhyay SK, Hursting SD, Morse HC. Splenic marginal zone B-cell and thymic T-cell lymphomas in p53-deficient mice. Lab Investig. 1999;79:3–14.
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7.
Maresch R, Mueller S, Veltkamp C, Öllinger R, Friedrich M, Heid I, et al. Multiplexed pancreatic genome engineering and cancer induction by transfection-based CRISPR/Cas9 delivery in mice. Nat Commun. 2016;7. https://doi.org/10.1038/ncomms10770.
van Zoest ID, Heijmen PS, Cruijsen PMJM, Jenks BG. Dynamics of background adaptation in Xenopus laevis: role of catecholamines and melanophore-stimulating hormone. Gen Comp Endocrinol. 1989;76:19–28.
Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004.
McEvoy J, Flores-Otero J, Zhang J, Nemeth K, Brennan R, Bradley C, et al. Coexpression of normally incompatible developmental pathways in retinoblastoma genesis. Cancer Cell. 2011;20:260–75.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20.
Steed TC, Treiber JM, Patel K, Ramakrishnan V, Merk A, Smith AR, et al. Differential localization of glioblastoma subtype: implications on glioblastoma pathogenesis. Oncotarget. 2016;7:24899–907.
Schaffer BE, Park K-S, Yiu G, Conklin JF, Lin C, Burkhart DL, et al. Loss of p130 accelerates tumor development in a mouse model for human small-cell lung carcinoma. Cancer Res. 2010;70:3877–83.
Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–84.
Abou-El-Ardat K, Seifert M, Becker K, Eisenreich S, Lehmann M, Hackmann K, et al. Comprehensive molecular characterization of multifocal glioblastoma proves its monoclonal origin and reveals novel insights into clonal evolution and heterogeneity of glioblastomas. Neuro Oncol. 2017;19:546–57.
Wiedemeyer WR, Dunn IF, Quayle SN, Zhang J, Chheda MG, Dunn GP, et al. Pattern of retinoblastoma pathway inactivation dictates response to CDK4/6 inhibition in GBM. Proc Natl Acad Sci USA. 2010;107:11501–6.
Schmid RS, Simon JM, Vitucci M, McNeill RS, Bash RE, Werneke AM, et al. Core pathway mutations induce de-differentiation of murine astrocytes into glioblastoma stem cells that are sensitive to radiation but resistant to temozolomide. Neuro Oncol. 2016;18:962–73.
Liu F, Gong J, Huang W, Wang Z, Wang M, Yang J, et al. MicroRNA-106b-5p boosts glioma tumorigensis by targeting multiple tumor suppressor genes. Oncogene. 2014;33:4813–22.
Vitucci M, Irvin DM, McNeill RS, Schmid RS, Simon JM, Dhruv HD, et al. Genomic profiles of low-grade murine gliomas evolve during progression to glioblastoma. Neuro Oncol. 2017;19:1237–47.
Wojton J, Chu Z, Mathsyaraja H, Meisen WH, Denton N, Kwon C-H, et al. Systemic delivery of SapC-DOPS has antiangiogenic and antitumor effects against glioblastoma. Mol Ther. 2013;21:1517–25.
Vanderluit JL, Wylie CA, McClellan KA, Ghanem N, Fortin A, Callaghan S, et al. The retinoblastoma family member p107 regulates the rate of progenitor commitment to a neuronal fate. J Cell Biol. 2007;178:129–39.
Pan D, Chen Y, Du Y, Ren Z, Li X, Hu B. Methylation of promoter of RBL1 enhances the radioresistance of three dimensional cultured carcinoma cells. Oncotarget. 2017;8:4422–35.
Harb G, Vasavada RC, Cobrinik D, Stewart AF. The retinoblastoma protein and its homolog p130 regulate the G1/S transition in pancreatic beta-cells. Diabetes. 2009;58:1852–62.
Cai EP, Luk CT, Wu X, Schroer SA, Shi SY, Sivasubramaniyam T, et al. Rb and p107 are required for alpha cell survival, beta cell cycle control and glucagon-like peptide-1 action. Diabetologia. 2014;57:2555–65.
Vasavada RC, Cozar-Castellano I, Sipula D, Stewart AF. Tissue-specific deletion of the retinoblastoma protein in the pancreatic beta-cell has limited effects on beta-cell replication, mass, and function. Diabetes. 2007;56:57–64.
Glenn ST, Jones CA, Sexton S, LeVea CM, Caraker SM, Hajduczok G, et al. Conditional deletion of p53 and Rb in the renin-expressing compartment of the pancreas leads to a highly penetrant metastatic pancreatic neuroendocrine carcinoma. Oncogene. 2014;33:5706–15.
Solin SL, Shive HR, Woolard KD, Essner JJ, McGrail M. Rapid tumor induction in zebrafish by TALEN-mediated somatic inactivation of the retinoblastoma1 tumor suppressor rb1. Sci Rep. 2015;5:13745.
Shim J, Choi J-H, Park M-H, Kim H, Kim JH, Kim S-Y, et al. Development of zebrafish medulloblastoma-like PNET model by TALEN-mediated somatic gene inactivation. Oncotarget. 2017;8:55280–97.
Jiang Z, Deng T, Jones R, Li H, Herschkowitz JI, Liu JC, et al. Rb deletion in mouse mammary progenitors induces luminal-B or basal-like/EMT tumor subtypes depending on p53 status. J Clin Investig. 2010;120:3296–309.
Shi Z, Xin H, Tian D, Lian J, Wang J, Liu G, et al. Modeling human point mutation diseases in Xenopus tropicalis with a modified CRISPR/Cas9 system. FASEB J. 2019;33:6962–8.
Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–57
Strecker J, Ladha A, Gardner Z, Schmid-Burgk JL, Makarova KS, Koonin EV, et al. RNA-guided DNA insertion with CRISPR-associated transposases. Science. 2019;365:48–53.
Naert T, Nieuwenhuysen T Van, Demuynck S, Grande S de, Przybyl J, Vuylsteke M, et al. CRISPR-NSID: an in vivo CRISPR/Cas9 negative selection screen reveals EZH2 as a druggable dependency factor in a genetic desmoid tumor model. 2019. https://www.biorxiv.org/content/10.1101/595769v1.
Moreno-Mateos MA, Vejnar CE, Beaudoin J-D, Fernandez JP, Mis EK, Khokha MK, et al. CRISPRscan: designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat Methods. 2015;12:982–8.
Naert T, Vleminckx K. Methods for CRISPR/Cas9 Xenopus tropicalis Tissue-Specific Multiplex Genome Engineering. Methods Mol Biol. 2018;1865:33–54.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034.1.
Acknowledgements
Research in the authors’ laboratory is supported by the Research Foundation—Flanders (FWO-Vlaanderen) (grants G0A1515N and G029413N), by the Belgian Science Policy (Interuniversity Attraction Poles—IAP7/07) and by the Concerted Research Actions from Ghent University (BOF15/GOA/011). Further support was obtained by the Hercules Foundation, Flanders (grant AUGE/11/14) and the Desmoid Tumor Research Foundation. TN is funded by “Kom op tegen Kanker” (Stand up to Cancer), the Flemish cancer society and previously held PhD fellowship with VLAIO-HERMES during the course of this work. DT and MC have a PhD fellowship from the Research Foundation—Flanders (FWO-Vlaanderen). We thank the Xenopus laevis Resource for Immunobiology (Rochester, NY, NIH R24 AI059830) for the kind gift of monoclonal antibody AM20 (10A91, CD8). We are indebted to Tim Deceuninck for animal care, Kelly Lemeire for technical assistance with TUNEL staining. We would like to thank the VIB BioImaging Core, and in particular Chris Guerin, Eef Parthoens, and Anneke Kremer, for access to the instrument park, training, and support.
Author information
Authors and Affiliations
Contributions
TN, DDi, and KV designed the study. DDi, TN, DT, and RN were involved in generation and phenotyping of the tp53 mutant X. tropicalis. TN performed genome engineering and phenotyping of all rb1/rbl1, rb1/rbl1/tp53, and rb1/rbl1/tp53/pten mutants. TN, LE, and DDe were involved in laser-capture microdissection and downstream analysis. DC and JvD performed pathological analysis. DT, DDi, and GvI performed flow cytometry experiments. CV performed X-ray imaging. SD provided technical assistance throughout the project. MC performed BATCH-GE analysis of targeted amplicon sequencing data. TN and KV wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naert, T., Dimitrakopoulou, D., Tulkens, D. et al. RBL1 (p107) functions as tumor suppressor in glioblastoma and small-cell pancreatic neuroendocrine carcinoma in Xenopus tropicalis. Oncogene 39, 2692–2706 (2020). https://doi.org/10.1038/s41388-020-1173-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-1173-z