Abstract
The Rad50 hook interface is crucial for assembly and various functions of the Mre11 complex. Previous analyses suggested that Rad50 molecules interact within (intracomplex) or between (intercomplex) dimeric complexes. In this study, we determined the structure of the human Rad50 hook and coiled-coil domains. The data suggest that the predominant structure is the intracomplex, in which the two parallel coiled coils proximal to the hook form a rod shape, and that a novel interface within the coiled-coil domains of Rad50 stabilizes the interaction of Rad50 protomers in the dimeric assembly. In yeast, removal of the coiled-coil interface compromised Tel1 activation without affecting DNA repair, while simultaneous disruption of that interface and the hook phenocopied a null mutation. The results demonstrate that the hook and coiled-coil interfaces coordinately promote intracomplex assembly and define the intracomplex as the functional form of the Mre11 complex.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Schiller, C.B. et al. Structure of Mre11-Nbs1 complex yields insights into ataxia-telangiectasia-like disease mutations and DNA damage signaling. Nat. Struct. Mol. Biol. 19, 693–700 (2012).
de Jager, M. et al. Differential arrangements of conserved building blocks among homologs of the Rad50/Mre11 DNA repair protein complex. J. Mol. Biol. 339, 937–949 (2004).
Hirano, T. At the heart of the chromosome: SMC proteins in action. Nat. Rev. Mol. Cell Biol. 7, 311–322 (2006).
Wyman, C., Lebbink, J. & Kanaar, R. Mre11-Rad50 complex crystals suggest molecular calisthenics. DNA Repair (Amst.) 10, 1066–1070 (2011).
Deshpande, R.A. et al. ATP-driven Rad50 conformations regulate DNA tethering, end resection, and ATM checkpoint signaling. EMBO J. 33, 482–500 (2014).
Liu, Y. et al. ATP-dependent DNA binding, unwinding, and resection by the Mre11/Rad50 complex. EMBO J. 35, 743–758 (2016).
Paull, T.T. & Gellert, M. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 13, 1276–1288 (1999).
Stracker, T.H. & Petrini, J.H. The MRE11 complex: starting from the ends. Nat. Rev. Mol. Cell Biol. 12, 90–103 (2011).
Lim, H.S., Kim, J.S., Park, Y.B., Gwon, G.H. & Cho, Y. Crystal structure of the Mre11-Rad50-ATPγS complex: understanding the interplay between Mre11 and Rad50. Genes Dev. 25, 1091–1104 (2011).
Möckel, C., Lammens, K., Schele, A. & Hopfner, K.P. ATP driven structural changes of the bacterial Mre11:Rad50 catalytic head complex. Nucleic Acids Res. 40, 914–927 (2012).
Rojowska, A. et al. Structure of the Rad50 DNA double-strand break repair protein in complex with DNA. EMBO J. 33, 2847–2859 (2014).
Seifert, F.U., Lammens, K., Stoehr, G., Kessler, B. & Hopfner, K.P. Structural mechanism of ATP-dependent DNA binding and DNA end bridging by eukaryotic Rad50. EMBO J. 35, 759–772 (2016).
Sung, S. et al. DNA end recognition by the Mre11 nuclease dimer: insights into resection and repair of damaged DNA. EMBO J. 33, 2422–2435 (2014).
Williams, R.S. et al. Mre11 dimers coordinate DNA end bridging and nuclease processing in double-strand-break repair. Cell 135, 97–109 (2008).
Lee, J.H. et al. Ataxia telangiectasia-mutated (ATM) kinase activity is regulated by ATP-driven conformational changes in the Mre11/Rad50/Nbs1 (MRN) complex. J. Biol. Chem. 288, 12840–12851 (2013).
Lammens, K. et al. The Mre11:Rad50 structure shows an ATP-dependent molecular clamp in DNA double-strand break repair. Cell 145, 54–66 (2011).
Williams, G.J. et al. ABC ATPase signature helices in Rad50 link nucleotide state to Mre11 interface for DNA repair. Nat. Struct. Mol. Biol. 18, 423–431 (2011).
Lafrance-Vanasse, J., Williams, G.J. & Tainer, J.A. Envisioning the dynamics and flexibility of Mre11-Rad50-Nbs1 complex to decipher its roles in DNA replication and repair. Prog. Biophys. Mol. Biol. 117, 182–193 (2015).
Barfoot, T. et al. Functional analysis of the bacteriophage T4 Rad50 homolog (gp46) coiled-coil domain. J. Biol. Chem. 290, 23905–23915 (2015).
Hohl, M. et al. Interdependence of the rad50 hook and globular domain functions. Mol. Cell 57, 479–491 (2015).
Hopfner, K.P. et al. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature 418, 562–566 (2002).
Wiltzius, J.J., Hohl, M., Fleming, J.C. & Petrini, J.H. The Rad50 hook domain is a critical determinant of Mre11 complex functions. Nat. Struct. Mol. Biol. 12, 403–407 (2005).
Roset, R. et al. The Rad50 hook domain regulates DNA damage signaling and tumorigenesis. Genes Dev. 28, 451–462 (2014).
Bressan, D.A., Baxter, B.K. & Petrini, J.H. The Mre11-Rad50-Xrs2 protein complex facilitates homologous recombination-based double-strand break repair in Saccharomyces cerevisiae. Mol. Cell. Biol. 19, 7681–7687 (1999).
Hohl, M. et al. The Rad50 coiled-coil domain is indispensable for Mre11 complex functions. Nat. Struct. Mol. Biol. 18, 1124–1131 (2011).
van der Linden, E., Sanchez, H., Kinoshita, E., Kanaar, R. & Wyman, C. RAD50 and NBS1 form a stable complex functional in DNA binding and tethering. Nucleic Acids Res. 37, 1580–1588 (2009).
Moreno-Herrero, F. et al. Mesoscale conformational changes in the DNA-repair complex Rad50/Mre11/Nbs1 upon binding DNA. Nature 437, 440–443 (2005).
Kochańczyk, T., Jakimowicz, P. & Krȩżel, A. Femtomolar Zn(II) affinity of minimal zinc hook peptides—a promising small tag for protein engineering. Chem. Commun. (Camb.) 49, 1312–1314 (2013).
Haering, C.H., Löwe, J., Hochwagen, A. & Nasmyth, K. Molecular architecture of SMC proteins and the yeast cohesin complex. Mol. Cell 9, 773–788 (2002).
Boulton, S.J. & Jackson, S.P. Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J. 17, 1819–1828 (1998).
Chen, L., Trujillo, K., Ramos, W., Sung, P. & Tomkinson, A.E. Promotion of Dnl4-catalyzed DNA end-joining by the Rad50/Mre11/Xrs2 and Hdf1/Hdf2 complexes. Mol. Cell 8, 1105–1115 (2001).
Moore, J.K. & Haber, J.E. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 2164–2173 (1996).
Keeney, S. Spo11 and the formation of DNA double-strand breaks in meiosis. Genome Dyn. Stab. 2, 81–123 (2008).
Usui, T., Ogawa, H. & Petrini, J.H. A DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol. Cell 7, 1255–1266 (2001).
Lee, J.H. & Paull, T.T. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 308, 551–554 (2005).
Lee, J.H. et al. Regulation of Mre11/Rad50 by Nbs1: effects on nucleotide-dependent DNA binding and association with ataxia-telangiectasia-like disorder mutant complexes. J. Biol. Chem. 278, 45171–45181 (2003).
Soh, Y.M. et al. Molecular basis for SMC rod formation and its dissolution upon DNA binding. Mol. Cell 57, 290–303 (2015).
Gatei, M. et al. ATM protein-dependent phosphorylation of Rad50 protein regulates DNA repair and cell cycle control. J. Biol. Chem. 286, 31542–31556 (2011).
de Jager, M. et al. Human Rad50/Mre11 is a flexible complex that can tether DNA ends. Mol. Cell 8, 1129–1135 (2001).
Tittel-Elmer, M. et al. Cohesin association to replication sites depends on rad50 and promotes fork restart. Mol. Cell 48, 98–108 (2012).
Unal, E. et al. DNA damage response pathway uses histone modification to assemble a double-strand break-specific cohesin domain. Mol. Cell 16, 991–1002 (2004).
Oh, J., Al-Zain, A., Cannavo, E., Cejka, P. & Symington, L.S. Xrs2 dependent and independent functions of the Mre11-Rad50 complex. Mol. Cell 64, 405–415 (2016).
Deshpande, R.A., Lee, J.H., Arora, S. & Paull, T.T. Nbs1 converts the human Mre11/Rad50 nuclease complex into an endo/exonuclease machine specific for protein-DNA adducts. Mol. Cell 64, 593–606 (2016).
Kozin, M.B. & Svergun, D.I. Automated matching of high- and low-resolution structural models. J. Appl. Crystallogr. 34, 33–41 (2001).
Svergun, D.I., Barberato, C. & Koch, M.H.J. CRYSOL—a program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates. J. Appl. Crystallogr. 28, 768–773 (1995).
Svergun, D.I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 25, 495–503 (1992).
Blanco-Canosa, J.B. & Dawson, P.E. An efficient Fmoc-SPPS approach for the generation of thioester peptide precursors for use in native chemical ligation. Angew. Chem. Int. Edn. Engl. 47, 6851–6855 (2008).
Lee, J.H. & Paull, T.T. Purification and biochemical characterization of ataxia-telangiectasia mutated and Mre11/Rad50/Nbs1. Methods Enzymol. 408, 529–539 (2006).
Bhaskara, V. et al. Rad50 adenylate kinase activity regulates DNA tethering by Mre11/Rad50 complexes. Mol. Cell 25, 647–661 (2007).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Martell, A.E. & Smith, R.M. Critical Stability Constants (Plenum Press, 1974).
Krężel, A. & Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 611, 3–19 (2016).
Alderighi, L. et al. Hyperquad simulation and speciation (HySS): a utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 184, 311–318 (1999).
Al-Ahmadie, H. et al. Synthetic lethality in ATM-deficient RAD50-mutant tumors underlies outlier response to cancer therapy. Cancer Discov. 4, 1014–1021 (2014).
Acknowledgements
We thank T. Kochańczyk for discussions and information regarding fluorescent protein modification, and members of the Cho and Petrini labs for helpful comments throughout. Expression constructs for wild-type MRN were gifts from T. Paull (University of Texas, Austin, Texas, USA). This work was supported by grants from the National Research Foundation of Korea (NRF) funded by the Korea Government (MEST, grant 2015R1A2A1A05001694 to Y.C.), the BK21 Program (Ministry of Education) (to Y.C.), the NIH (RO1 grant GM56888 to J.H.J.P.), the MSK Cancer Center (Core Grant P30 CA008748 to J.H.J.P.), and the National Science Center, Poland (Opus, grant 2014/13/B/NZ1/ 00935 to A.K.).
Author information
Authors and Affiliations
Contributions
Y.B.P. carried out crystallization and structure determination; Y.B.P., M.P., M.H. and E.J. participated in biochemical experiments; K.S.J. performed SAXS analyses; M.H. and J.H.J.P. designed and performed yeast genetics experiments; Y.C. and J.H.J.P. designed research in consultation with A.K.; and J.H.J.P., Y.C., A.K. and M.H. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
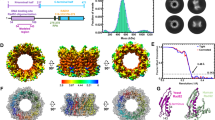
Supplementary Figure 1 Structure of the human and yeast Rad50 hook and coiled-coil domains.
(a) Analytical ultracentrifuge data of the Zn-free (bottom left) and –bound Rad50HCC182 constructs (bottom right). (b) Stereoview of the section of the electron density map of Rad50HCC182. The 2.8 Å experimental map contoured at 1.2 I/σ was generated with phases from derived Se-Met anomalous scattering. (c) Sequence alignment of Rad50 homologs. Secondary structures of the coiled-coil domain of HsRad50 are represented above the sequence. Secondary structures of PfRad50 are shown below the sequence of PfRad50. Conserved residues in Rad50 proteins are marked with a yellow box. Mutations identified in breast cancer patients are marked with a blue star. Residues involved in dimerization are marked with a violet square. Rad50 hook mutant suppressors20 are marked with a green triangle. Every tenth residue is marked with a black circle. (d-e) A model for ScRad50 apex dimer. (d) The model was generated using the program Modeller (Sali, A. & Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993)) based on human Rad50 apex structure. Close up view of the hook interface of ScRad50 shown in the same orientation as in Fig. 2a. (e) Close up view of the hook and coiled-coil interfaces of ScRad50. The view is orthogonal from that of Supplementary Fig.1d. (f) Close up view of the coiled-coil interface of ScRad50.
Supplementary Figure 2 The Rad50 ‘hinge’ loop and hook domain cooperate to promote DSB repair and Mre11-complex integrity.
(a) Close up view of the hinge loop in Rad50 A (top, blue) and Rad50B (bottom, orange). The three mutated residues are highlighted in yellow. (b, c) DNA damage sensitivity of the mutants towards MMS (b) and CPT (c). Two independent strains of rad50-48-PPP are shown. All strains were integrated at their native locus, except rad50-PPP was expressed plasmid based in a rad50Δ strain (marked by red asterisk). Plates were incubated at 30°C. Strains with rad50ΔCC and rad50ΔCC-48 were included as reference. (d) DNA damage sensitivity of the mutants in a mec1Δ strain or a mec1Δ sae2Δ strain towards MMS. (e) Mre11 complex integrity in rad50-PPP “hinge loop” and rad50-48 single and double mutants assessed by co-immunoprecipitation and Western blot with Rad50 or Mre11 antisera.
Supplementary Figure 3 Solution structure of the PfRad50HCC dimer and models for the ‘hook’ and ‘coiled-coil’ mutants.
(a) Molecular envelope of the PfRad50HCC dimer (residues 351-532) calculated from the SAXS data. (b) X-ray scattering profile of PfRad50HCC protein in solution, which was measured at 4°C. The open symbols are the experimental data and the solid line is the X-ray scattering profile. The Guinier plot of PfRad50HCC protein is shown in the inset. (c) Pair distance distribution p(r) function for PfRad50HCC protein, based on an analysis of the experimental SAXS data (empty circles) using the program GNOM56. (d) A model for the SMC hinge mutant. T. maritima SMC hinge domain (residues 475 to 679, magenta and cyan) was fused to HsRad50 coiled-coils (585-651 and 714-766, blue and orange) at N- and C-terminal end, respectively. Detailed construct information is in Supplementary Table 4. (e) A Pfhook mutant dimer model. For FRET analysis, hook domain of PfRad50 (421-476, green and magenta for each monomer) is fused to 651-656 and 715-722 of HsRad50 at N- and C-terminal domain, respectively. (f) A Pfhook monomer model. The hook domain of PfRad50 (421-476, green) is fused to HsRad50 coiled-coils (585-656) and (715 to 766) at N- and C-terminal end, respectively. Gel filtration and ultracentrifuge analyses reveal that this construct favors monomer formation. (g) A model of coiled-coil deletion mutant of Rad50HCC182 (ΔCC mutant). Residues 614-668 and 702-750 are deleted. Residues (585-613, 751-766) of each monomer are shown in light blue and light orange, respectively, and junctions are marked.
Supplementary Figure 4 Gel filtration and circular dichroism analysis of the wild-type and Rad50 apex mutants.
(a) Gel filtration analysis of Rad50HCC182 WT and mutants; Wild-type (blue), SMC hinge (red), Pfhook (green), ΔCC (brown), and ΔCC-48 (magenta). Standard molecular weight markers are marked on top of the graph. The profile for WT Rad50HCC182 in absence of Zn2+ is identical to that in the presence of Zn2+ and omitted for clarity. (b) Circular dichroism analysis of the HsRad50HCC182 WT and “hook” and “coiled-coil” mutants; Wild-type (black), Pfhook (green), ΔCC (red), and ΔCC-48 (blue). Various HsRad50 mutants are monitored by CD spectrophotometer (Jasco J-715) at wavelength of 200-260 nm. We did not examine the SMC hinge mutant because of the large differences in hinge region compared to the hook domain. (c) Analytical ultracentrifuge data of the SMC hinge, Pfhook, ΔCC, and ΔCC-48 (shown from left to right).
Supplementary Figure 5 Telomere lengths in yeast mutants and DNA-binding activities of various HsMRN complexes.
(a) rad50 mutants are defective in telomere maintenance. Telomere length after 0, 15 and 30 generations of growth (0, 1 and 2 days) at 30°C. Heterozygote diploids (RAD50/rad50hook) were included as 0 generations of growth. Genomic DNA was digested with PstI and telomeres were visualized by Southern blot using a telomere specific probe. (b-e) DNA binding activities of various HsMRN complexes. EMSA in the presence of AMPPNP with 0 (lane 1), 100 (lane 2), 200 (lane 3), 400 (lane 4), 600 (lane 5) and 800 nM of WT MRN (lane 6). (b)The WT MRN complex (800 nM) was incubated without AMPPNP in lane 7, (c) MRN with Rad50ΔCC, (d) MRN with Rad50 Pfhook mutant, (e) MRN with Rad50ΔCC-48.
Supplementary Figure 6 Modeling of the yeast rad50-46 coiled-coil-domain suppressor N607Y.
The N607Y suppressor mutant at the coiled-coil of yeast Rad5020 was modeled based on the crystal structure of human Rad50. Two closely located side chains of Tyr607 from the Rad50 dimer may facilitate the coiled-coil interactions between the Rad50 dimer.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Tables 1–5 and Supplementary Notes 1–2 (PDF 1460 kb)
Supplementary Data Set 1
Uncropped gel data for Fig 4j-m (PDF 5497 kb)
Rights and permissions
About this article
Cite this article
Park, Y., Hohl, M., Padjasek, M. et al. Eukaryotic Rad50 functions as a rod-shaped dimer. Nat Struct Mol Biol 24, 248–257 (2017). https://doi.org/10.1038/nsmb.3369
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3369
This article is cited by
-
Mechanism of MRX inhibition by Rif2 at telomeres
Nature Communications (2021)
-
Analysis of the impact of three phthalates on the freshwater gastropod Physella acuta at the transcriptional level
Scientific Reports (2021)
-
Crystal structure of the nuclease and capping domain of SbcD from Staphylococcus aureus
Journal of Microbiology (2021)
-
DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer
Signal Transduction and Targeted Therapy (2020)
-
The condensin holocomplex cycles dynamically between open and collapsed states
Nature Structural & Molecular Biology (2020)