Abstract
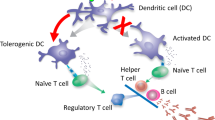
The development of antidrug antibodies (ADAs) is a common cause for the failure of biotherapeutic treatments and adverse hypersensitivity reactions. Here we demonstrate that poly(lactic-co-glycolic acid) (PLGA) nanoparticles carrying rapamycin, but not free rapamycin, are capable of inducing durable immunological tolerance to co-administered proteins that is characterized by the induction of tolerogenic dendritic cells, an increase in regulatory T cells, a reduction in B cell activation and germinal centre formation, and the inhibition of antigen-specific hypersensitivity reactions. Intravenous co-administration of tolerogenic nanoparticles with pegylated uricase inhibited the formation of ADAs in mice and non-human primates and normalized serum uric acid levels in uricase-deficient mice. Similarly, the subcutaneous co-administration of nanoparticles with adalimumab resulted in the durable inhibition of ADAs, leading to normalized pharmacokinetics of the anti-TNFα antibody and protection against arthritis in TNFα transgenic mice. Adjunct therapy with tolerogenic nanoparticles represents a novel and broadly applicable approach to prevent the formation of ADAs against biologic therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Goodman, M. Market watch: sales of biologics to show robust growth through to 2013. Nat. Rev. Drug Discov. 8, 837 (2009).
Sathish, J. G. et al. Challenges and approaches for the development of safer immunomodulatory biologics. Nat. Rev. Drug Discov. 12, 306–324 (2013).
van Schouwenburg, P. A., Rispens, T. & Wolbink, G. J. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat. Rev. Rheumatol. 9, 164–172 (2013).
Rosenberg, A. S. Immunogenicity of biological therapeutics: a hierarchy of concerns. Dev. Biol. 112, 15–21 (2003).
Schellekens, H. The immunogenicity of therapeutic proteins. Discov. Med. 9, 560–564 (2010).
Chirmule, N., Jawa, V. & Meibohm, B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPS J. 14, 296–302 (2012).
Casadevall, N. et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N. Engl. J. Med. 346, 469–475 (2002).
Berrier, K. L. et al. CRIM-negative infantile Pompe disease: characterization of immune responses in patients treated with ERT monotherapy. Genet. Med. 17, 912–918 (2015).
Bartelds, G. M. et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 305, 1460–1468 (2011).
Schellekens, H., Hennink, W. E. & Brinks, V. The immunogenicity of polyethylene glycol: facts and fiction. Pharm. Res. 30, 1729–1734 (2013).
Sundy, J. S. et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA 306, 711–720 (2011).
Lipsky, P. E. et al. Pegloticase immunogenicity: the relationship between efficacy and antibody development in patients treated for refractory chronic gout. Arthritis Res. Ther. 16, R60 (2014).
Parenky, A. et al. New FDA draft guidance on immunogenicity. AAPS J. 16, 499–503 (2014).
Krieckaert, C. L., Bartelds, G. M. & Wolbink, G. J. Therapy: Immunogenicity of biologic therapies-we need tolerance. Nat. Rev. Rheumatol. 6, 558–559 (2010).
Maldonado, R. A. & von Andrian, U. H. How tolerogenic dendritic cells induce regulatory T cells. Adv. Immunol. 108, 111–165 (2010).
Naranjo-Gomez, M. et al. Comparative study of clinical grade human tolerogenic dendritic cells. J. Transl. Med. 9, 89 (2011).
Fischer, R., Turnquist, H. R., Taner, T. & Thomson, A. W. Use of rapamycin in the induction of tolerogenic dendritic cells. Handb Exp Pharmacol, 215–232 (2009).
Maldonado, R. A. et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl Acad. Sci. USA 112, E156–E165 (2015).
Wu, X. et al. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc. Natl Acad. Sci. USA 91, 742–746 (1994).
Haile, L. A., Puig, M., Kelley-Baker, L. & Verthelyi, D. Detection of innate immune response modulating impurities in therapeutic proteins. PLoS ONE 10, e0125078 (2015).
Joseph, A., Munroe, K., Housman, M., Garman, R. & Richards, S. Immune tolerance induction to enzyme-replacement therapy by co-administration of short-term, low-dose methotrexate in a murine Pompe disease model. Clin. Exp. Immunol. 152, 138–146 (2008).
Barnden, M. J., Allison, J., Heath, W. R. & Carbone, F. R. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell. Biol. 76, 34–40 (1998).
Merrell, K. T. et al. Identification of anergic B cells within a wild-type repertoire. Immunity 25, 953–962 (2006).
Matsumoto, M. et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 41, 1040–1051 (2014).
Bomalaski, J. S., Holtsberg, F. W., Ensor, C. M. & Clark, M. A. Uricase formulated with polyethylene glycol (uricase-PEG 20): biochemical rationale and preclinical studies. J. Rheumatol. 29, 1942–1949 (2002).
Hayward, M. D. et al. An extensive phenotypic characterization of the hTNFalpha transgenic mice. BMC Physiol. 7, 13 (2007).
Hamilton, J. A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8, 533–544 (2008).
Irvine, D. J., Swartz, M. A. & Szeto, G. L. Engineering synthetic vaccines using cues from natural immunity. Nature Mater. 12, 978–990 (2013).
Metcalfe, S. M. & Fahmy, T. M. Targeted nanotherapy for induction of therapeutic immune responses. Trends Mol. Med. 18, 72–80 (2012).
Bachmann, M. F. & Jennings, G. T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 10, 787–796 (2010).
Donahue, A. C. & Fruman, D. A. Distinct signaling mechanisms activate the target of rapamycin in response to different B-cell stimuli. Eur. J. Immunol. 37, 2923–2936 (2007).
Limon, J. J. & Fruman, D. A. Akt and mTOR in B cell activation and differentiation. Front. Immunol. 3, 228 (2012).
Keating, R. et al. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nature Immunol. 14, 1266–1276 (2013).
Zhao, Y. et al. Rapamycin prevents bronchiolitis obliterans through increasing infiltration of regulatory B cells in a murine tracheal transplantation model. J. Thorac. Cardiovasc. Surg. 151, 487–496 (2016).
Pasut, G. & Veronese, F. M. State of the art in PEGylation: the great versatility achieved after forty years of research. J. Control. Release 161, 461–472 (2012).
Armstrong, J. K. et al. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer 110, 103–111 (2007).
De Groot, A. S., Knopp, P. M. & Martin, W. De-immunization of therapeutic proteins by T-cell epitope modification. Dev. Biol. 122, 171–194 (2005).
Bryson, C. J., Jones, T. D. & Baker, M. P. Prediction of immunogenicity of therapeutic proteins: validity of computational tools. BioDrugs 24, 1–8 (2010).
Onda, M. Reducing the immunogenicity of protein therapeutics. Curr. Drug Targets 10, 131–139 (2009).
Mazor, R., Tai, C. H., Lee, B. & Pastan, I. Poor correlation between T-cell activation assays and HLA-DR binding prediction algorithms in an immunogenic fragment of Pseudomonas exotoxin A. J. Immunol. Methods 425, 10–20 (2015).
Mazor, R. et al. Recombinant immunotoxin for cancer treatment with low immunogenicity by identification and silencing of human T-cell epitopes. Proc. Natl Acad. Sci. USA 111, 8571–8576 (2014).
Macauley, M. S. & Paulson, J. C. Siglecs induce tolerance to cell surface antigens by BIM-dependent deletion of the antigen-reactive B cells. J. Immunol. 193, 4312–4321 (2014).
Scott, D. W. Inhibitors – cellular aspects and novel approaches for tolerance. Haemophilia 20(Suppl 4), 80–86 (2014).
Lorentz, K. M., Kontos, S., Diaceri, G., Henry, H. & Hubbell, J. A. Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase. Sci. Adv. 1, e1500112 (2015).
Gilliland, L. K. et al. Elimination of the immunogenicity of therapeutic antibodies. J. Immunol. 162, 3663–3671 (1999).
Colowick, A. B., Bohn, R. L., Avorn, J. & Ewenstein, B. M. Immune tolerance induction in hemophilia patients with inhibitors: costly can be cheaper. Blood 96, 1698–1702 (2000).
Mendelsohn, N. J., Messinger, Y. H., Rosenberg, A. S. & Kishnani, P. S. Elimination of antibodies to recombinant enzyme in Pompe's disease. N. Engl. J. Med. 360, 194–195 (2009).
Safety and Pharmacodynamics of SEL-212 (Pegsiticase+SEL-110) in Subjects With Elevated Blood Uric Acid Levels. ClinicalTrials.gov. http://dx.doi.org/NCT02648269.
Astete, C. E. & Sabliov, C. M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 17, 247–289 (2006).
Liu, E. et al. Anti-peptide autoantibodies and fatal anaphylaxis in NOD mice in response to insulin self-peptides B:9-23 and B:13-23. J. Clin. Invest. 110, 1021–1027 (2002).
Acknowledgements
The authors gratefully acknowledge the excellent support of M. Allen, M. Mwanza and B. Woldemeskel for ELISA analyses, R. Damien for the analytical characterization of nanoparticles and Y. Gao for the preparation of the KLH-PEG. We also thank M. Schwartzschild for providing the uricase-deficient mice.
Author information
Authors and Affiliations
Contributions
T.K.K., J.D.F., R.A.L., P.N.K and R.A.M. designed the in vivo and ex vivo studies and analysed data, A.P.G., C.O'N., D.H.A., and L.J. designed and developed the nanoparticles, E.B. and A.C. developed the ELISA methods and analysed data, V.C. developed the method for analysis of uric acid, V.C., W.K., F.F., and N.V. developed the analytical methods to characterize the nanoparticles and analysed data, and T.K.K. and R.A.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors are employees and shareholders of Selecta Biosciences.
Supplementary information
Supplementary information
Supplementary information (PDF 583 kb)
Supplementary information
Supplementary information (PPTX 11461 kb)
Rights and permissions
About this article
Cite this article
Kishimoto, T., Ferrari, J., LaMothe, R. et al. Improving the efficacy and safety of biologic drugs with tolerogenic nanoparticles. Nature Nanotech 11, 890–899 (2016). https://doi.org/10.1038/nnano.2016.135
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2016.135
This article is cited by
-
Immunomodulatory potential of rapamycin-loaded mesoporous silica nanoparticles: pore size-dependent drug loading, release, and in vitro cellular responses
Drug Delivery and Translational Research (2024)
-
Targeted modulation of immune cells and tissues using engineered biomaterials
Nature Reviews Bioengineering (2023)
-
Phase 2 Dose-Finding Study in Patients with Gout Using SEL-212, a Novel PEGylated Uricase (SEL-037) Combined with Tolerogenic Nanoparticles (SEL-110)
Rheumatology and Therapy (2023)
-
Tolerogenic nanoparticles mitigate the formation of anti-drug antibodies against pegylated uricase in patients with hyperuricemia
Nature Communications (2022)
-
Enhancing the Response Rate to Recombinant Uricases in Patients with Gout
BioDrugs (2022)