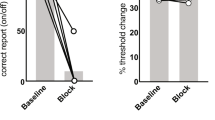
Abstract
The skin is equipped with specialized mechanoreceptors that allow the perception of the slightest brush. Indeed, some mechanoreceptors can detect even nanometer-scale movements. Movement is transformed into electrical signals via the gating of mechanically activated ion channels at sensory endings in the skin. The sensitivity of Piezo mechanically gated ion channels is controlled by stomatin-like protein-3 (STOML3), which is required for normal mechanoreceptor function. Here we identify small-molecule inhibitors of STOML3 oligomerization that reversibly reduce the sensitivity of mechanically gated currents in sensory neurons and silence mechanoreceptors in vivo. STOML3 inhibitors in the skin also reversibly attenuate fine touch perception in normal mice. Under pathophysiological conditions following nerve injury or diabetic neuropathy, the slightest touch can produce pain, and here STOML3 inhibitors can reverse mechanical hypersensitivity. Thus, small molecules applied locally to the skin can be used to modulate touch and may represent peripherally available drugs to treat tactile-driven pain following neuropathy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
09 January 2017
When this article was first published online, a version of the reporting checklist supplementary file with information pertaining to a previous version of this manuscript, which does not match the information presented in the published article, was posted by mistake. As of 9 January 2017, this mismatched file has been replaced by a file that matches the information in the article.
References
Bensmaia, S.J. Tactile intensity and population codes. Behav. Brain Res. 190, 165–173 (2008).
Poole, K., Herget, R., Lapatsina, L., Ngo, H.D. & Lewin, G.R. Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat. Commun. 5, 3520 (2014).
Woo, S.-H. et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626 (2014).
Ranade, S.S. et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125 (2014).
Moshourab, R.A., Wetzel, C., Martinez-Salgado, C. & Lewin, G.R. Stomatin-domain protein interactions with acid-sensing ion channels modulate nociceptor mechanosensitivity. J. Physiol. (Lond.) 591, 5555–5574 (2013).
Wetzel, C. et al. A stomatin-domain protein essential for touch sensation in the mouse. Nature 445, 206–209 (2007).
Costigan, M., Scholz, J. & Woolf, C.J. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 32, 1–32 (2009).
von Hehn, C.A., Baron, R. & Woolf, C.J. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73, 638–652 (2012).
Tal, M. & Bennett, G.J. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain 57, 375–382 (1994).
Brand, J. et al. A stomatin dimer modulates the activity of acid-sensing ion channels. EMBO J. 31, 3635–3646 (2012).
Chesler, A.T. et al. The role of PIEZO2 in human mechanosensation. N. Engl. J. Med. 375, 1355–1364 (2016).
Mahmud, A.A. et al. Loss of the proprioception and touch sensation channel PIEZO2 in siblings with a progressive form of contractures. Clin. Genet. http://dx.doi.org/10.1111/cge.12850 (2016).
Wang, Y. & Morrow, J.S. Identification and characterization of human SLP-2, a novel homologue of stomatin (band 7.2b) present in erythrocytes and other tissues. J. Biol. Chem. 275, 8062–8071 (2000).
Boute, N. et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24, 349–354 (2000).
Mairhofer, M., Steiner, M., Salzer, U. & Prohaska, R. Stomatin-like protein-1 interacts with stomatin and is targeted to late endosomes. J. Biol. Chem. 284, 29218–29229 (2009).
Da Cruz, S. et al. SLP-2 interacts with prohibitins in the mitochondrial inner membrane and contributes to their stability. Biochim. Biophys. Acta 1783, 904–911 (2008).
Lapatsina, L., Brand, J., Poole, K., Daumke, O. & Lewin, G.R. Stomatin-domain proteins. Eur. J. Cell Biol. 91, 240–245 (2012).
Hu, C.-D., Chinenov, Y. & Kerppola, T.K. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell 9, 789–798 (2002).
Heilemann, M. et al. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew. Chem. Int. Edn Engl. 47, 6172–6176 (2008).
Lampe, A., Haucke, V., Sigrist, S.J., Heilemann, M. & Schmoranzer, J. Multi-colour direct STORM with red emitting carbocyanines. Biol. Cell 104, 229–237 (2012).
Wolter, S. et al. rapidSTORM: accurate, fast open-source software for localization microscopy. Nat. Methods 9, 1040–1041 (2012).
Hu, J. & Lewin, G.R. Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J. Physiol. (Lond.) 577, 815–828 (2006).
McCarter, G.C., Reichling, D.B. & Levine, J.D. Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neurosci. Lett. 273, 179–182 (1999).
Price, M.P., Thompson, R.J., Eshcol, J.O., Wemmie, J.A. & Benson, C.J. Stomatin modulates gating of acid-sensing ion channels. J. Biol. Chem. 279, 53886–53891 (2004).
Kozlenkov, A., Lapatsina, L., Lewin, G.R. & Smith, E.S.J. Subunit-specific inhibition of acid sensing ion channels by stomatin-like protein 1. J. Physiol. (Lond.) 592, 557–569 (2014).
Milenkovic, N., Wetzel, C., Moshourab, R. & Lewin, G.R. Speed and temperature dependences of mechanotransduction in afferent fibers recorded from the mouse saphenous nerve. J. Neurophysiol. 100, 2771–2783 (2008).
Lewin, G.R. & Moshourab, R. Mechanosensation and pain. J. Neurobiol. 61, 30–44 (2004).
Beggs, S., Trang, T. & Salter, M.W. P2X4R+ microglia drive neuropathic pain. Nat. Neurosci. 15, 1068–1073 (2012).
Chaplan, S.R., Bach, F.W., Pogrel, J.W., Chung, J.M. & Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994).
Baron, R. Neuropathic pain: a clinical perspective. Handb. Exp. Pharmacol. 194, 3–30 (2009).
Sorge, R.E. et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 18, 1081–1083 (2015).
Dworkin, R.H. et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin. Proc. 85 (Suppl. 3), S3–S14 (2010).
Basbaum, A.I., Gautron, M., Jazat, F., Mayes, M. & Guilbaud, G. The spectrum of fiber loss in a model of neuropathic pain in the rat: an electron microscopic study. Pain 47, 359–367 (1991).
Decosterd, I. & Woolf, C.J. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87, 149–158 (2000).
Shields, S.D., Eckert, W.A. III & Basbaum, A.I. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J. Pain 4, 465–470 (2003).
Lewin, G.R., Lechner, S.G. & Smith, E.S.J. Nerve growth factor and nociception: from experimental embryology to new analgesic therapy. Handb. Exp. Pharmacol. 220, 251–282 (2014).
Lewin, G.R., Ritter, A.M. & Mendell, L.M. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J. Neurosci. 13, 2136–2148 (1993).
Lewin, G.R., Rueff, A. & Mendell, L.M. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur. J. Neurosci. 6, 1903–1912 (1994).
Korndörfer, I.P. & Skerra, A. Improved affinity of engineered streptavidin for the Strep-tag II peptide is due to a fixed open conformation of the lid-like loop at the binding site. Protein Sci. 11, 883–893 (2002).
Lapatsina, L. et al. Regulation of ASIC channels by a stomatin/STOML3 complex located in a mobile vesicle pool in sensory neurons. Open Biol. 2, 120096 (2012).
van Hecke, O., Austin, S.K., Khan, R.A., Smith, B.H. & Torrance, N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 155, 654–662 (2014).
Bierhaus, A. et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat. Med. 18, 926–933 (2012).
Abraira, V.E. & Ginty, D.D. The sensory neurons of touch. Neuron 79, 618–639 (2013).
Lechner, S.G. & Lewin, G.R. Hairy sensation. Physiology (Bethesda) 28, 142–150 (2013).
Syeda, R. et al. Chemical activation of the mechanotransduction channel Piezo1. eLife http://dx.doi.org/10.7554/eLife.07369 (2015).
Li, J. et al. Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282 (2014).
Ranade, S.S. et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA 111, 10347–10352 (2014).
Woo, S.-H. et al. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 18, 1756–1762 (2015).
Usoskin, D. et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153 (2015).
Lechner, S.G. & Lewin, G.R. Peripheral sensitisation of nociceptors via G-protein-dependent potentiation of mechanotransduction currents. J. Physiol. (Lond.) 587, 3493–3503 (2009).
Chiang, L.-Y. et al. Laminin-332 coordinates mechanotransduction and growth cone bifurcation in sensory neurons. Nat. Neurosci. 14, 993–1000 (2011).
Lechner, S.G., Frenzel, H., Wang, R. & Lewin, G.R. Developmental waves of mechanosensitivity acquisition in sensory neuron subtypes during embryonic development. EMBO J. 28, 1479–1491 (2009).
Brideau, C., Gunter, B., Pikounis, B. & Liaw, A. Improved statistical methods for hit selection in high-throughput screening. J. Biomol. Screen. 8, 634–647 (2003).
von Kleist, L. et al. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146, 471–484 (2011).
Vareniuk, I., Pavlov, I.A. & Obrosova, I.G. Inducible nitric oxide synthase gene deficiency counteracts multiple manifestations of peripheral neuropathy in a streptozotocin-induced mouse model of diabetes. Diabetologia 51, 2126–2133 (2008).
Osoegawa, K. et al. Bacterial artificial chromosome libraries for mouse sequencing and functional analysis. Genome Res. 10, 116–128 (2000).
Lee, E.C. et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73, 56–65 (2001).
Liu, P., Jenkins, N.A. & Copeland, N.G. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 13, 476–484 (2003).
Bunting, M., Bernstein, K.E., Greer, J.M., Capecchi, M.R. & Thomas, K.R. Targeting genes for self-excision in the germ line. Genes Dev. 13, 1524–1528 (1999).
Acknowledgements
We thank K. Barda, M. Braunschweig and H. Thraenhardt for technical assistance and G. Lichtner for providing the custom-written algorithm for dSTORM drift correction. We thank H.Wende for providing help and advice for generating the Stoml3lacZ allele and S. Lechner for comments on the MS. We also thank B. Purfürst for electron microscopy experiments. This study was funded by DFG collaborative research grant SFB958 (projects A09 to K.P. and G.R.L., A01 to V.H. and Z02 to J.S.). Additional support was provided by a senior ERC grant (grant number 294678 to G.R.L.) and by the NeuroCure Cluster of Excellence (to V.H., G.R.L. and J.F.A.P.). K.P. was supported by a Cecile-Vogt Fellowship (MDC). S.P. was supported by a Marie Curie Fellowship from the European Union (grant number 253663 Touch in situ). C.P. received a Ph.D. fellowship from the University of Cagliari. J.F.A.P. was funded by a European Research Council (ERC) starting grant (ERC-2010-StG-260590), the DFG (FOR 1341, FOR 2143), the Berlin Institute of Health (BIH) and the European Union (FP7, 3x3Dimaging 323945). R.K. was supported by an ERC Advanced Investigator grant (294293-PAIN PLASTICITY). D.H. was funded by the Berlin Institute of Health (BIH). E.St.J.S., L.E. and M.M. were supported by an Alexander von Humboldt Fellowship.
Author information
Authors and Affiliations
Contributions
K.P. designed and carried out the screen and characterized small molecules with dSTORM and patch clamp electrophysiology. C.W. performed ex vivo skin electrophysiology and experiments in mice and behavioral experiments. S.P. screened OB-1 for effects on mechanosensitive currents in DRGs. C.P. performed behavioral and real-time PCR experiments and performed histochemical analysis of the Stoml3lacZ mice. C.G. determined IC50s using the pili method. D.H. performed touch perception assays with L.E., who established the methodology. K.K.B. and R.K. established the diabetic neuropathy model and performed and analyzed behavioral experiments. A.L. and K.P. performed and analyzed dSTORM experiments. L.L., V.B., K.P., C.P. and J.W. generated and characterized the Stoml3lacZ and Stoml3StrepII mice. L.L. and R.F. performed molecular cloning experiments. E.St.J.S. performed ASIC experiments. M.M. performed additional electrophysiological experiments. J.K. analyzed transmission electron microscopy data. E.S. synthesized molecules and managed compound libraries. M.N. performed statistical analyses of high-throughput screening data and helped in design and execution of the screen. J.P.v.K. supervised screening experiments. J.F.A.P. established touch-perception assays and supervised the acquisition and analysis of the data. V.H. and J.S. directed and supervised imaging experiments. K.P., C.W. and G.R.L. wrote the paper. K.P., C.W. and G.R.L. conceived and directed the project.
Corresponding authors
Ethics declarations
Competing interests
G.R.L., K.P., C.W. and L.L. are named as inventors on a patent application related to data in this paper.
Integrated supplementary information
Supplementary Figure 1 Effects of STOML3-modulating molecules on other stomatin-domain proteins
(a) BiFC signal development observed when cells were transfected with stomatin –VC/-VN, STOML1-VC/-VN, STOML2-VC/-VN, STOML3-VC/-VN or podocin-VC/-VN expression constructs respectively. The slope of signal development was used as a measure of oligomerization. Note that OB-1 significantly inhibits oligomerization of stomatin, STOML1, STOML2 and STOML3 but does not affect podocin oligomerization. Unpaired t-test: stomatin vs. stomatin + OB-1, P < 0.0001; STOML1 vs. STOML1 + OB-1, P < 0.0001; STOML2 vs. STOML2 + OB-1, P < 0.0001; STOML3 vs. STOML3+ OB-1, P < 0.0001; Mann-Whitney U-test: podocin vs. podocin + OB-1, P = 0.0584; numbers indicate replicates derived from 2 independent cell transfections; data are shown as individual slopes and mean ± s.e.m.. (b) OB-1 was tested at two different concentrations (2 μM and 20 μM) using the BiFC assay. The normalized slope was significantly reduced when OB-1 was present. Unpaired t-test: vehicle vs. 20 μm OB-1, P < 0.0001; vehicle vs. 2 μm OB-1, P < 0.0001; numbers indicate replicates derived from two independent cell transfections; data are displayed as individual slopes and mean ± s.e.m. *** P < 0.001; ns nonsignificant.
Supplementary Figure 2 Stoml3 mRNA levels after inhibitor treatment and quantitative analysis of mechanically gated currents.
(a) N2a cells and acutely isolated DRGs were treated with either vehicle or OB-1 (20 μM) for 3 hours before mRNA was isolated, reverse transcribed and analyzed using qPCR. There were no detectable differences in Stoml3 transcript levels; numbers indicate independent RNA preparations derived from 3 N2a cell preparations and from two mice per DRG preparation; data are displayed as individual data points and mean ± s.e.m.. (b) Time course analysis of OB-1 activity in N2a cells. Cells were treated with 20 μM OB-1 for the indicated time and mechanotransduction was monitored using pillar arrays. Treatment of at least 3 hours was required for maximum inhibition of mechanically-gated currents. Mann-Whitney U-test: 0-1h vs. > 3h OB-1, P = 0.0003. Unpaired t-test: 1-2h vs. > 3h OB-1, P = 0.0033; 2-3h vs. >3h OB-1, P = 0.0132; numbers indicate currents measured from N2a cells derived from more than 6 independent experiments; data are shown as current amplitude, each bin is displayed as mean of cell averages ± s.e.m.. (c) Representative current trace with latency (magenta) activation time constant (τ1, blue) and inactivation time constant (τ2, green) indicated by dashed lines. The inactivation time constant of mechanically gated currents in DRG mechanoreceptors (mec) shown here was measured using elastomeric pillar arrays. There is a trend for longer inactivation time constants that is not significant. Mann-Whitney U-test: mec vs. mec + OB-1, P = 0.1966; mec vs. mec + OB-2, P = 0.7930; data are displayed as box and whisker plot, fit of inactivation of individual curve, displayed as median with 5-95 interquartiles. (d) In nociceptors (noci) treatment with OB-2 led to significantly longer latencies and significantly slower activation time constants compared to non-treated noci. Mann-Whitney U-test: vehicle vs. OB-2 (Latency), P = 0.0315; vehicle vs. OB-2 (activation time constant), P = 0.001; data are displayed as box and whisker plot, fit of inactivation of individual curve, displayed as median with 5-95 interquartiles. * P < 0.05; ** P < 0.01; *** P < 0.001.
Supplementary Figure 3 Effects of OB-1 on mechanically-activated currents in mouse sensory neurons.
(a) Schematic diagram of a large diameter mouse sensory neuron with recording electrode (RE) and nanostimulator (NS). (b) Typical RA-mechanosensitive current evoked from mouse sensory neurons by poking the cell soma. (c) Typical RA-mechanosensitive currents evoked from neurons pre-incubated with OB-1 or vehicle. Red lines indicate the exponential fit of the current inactivation to determine τ2. (d) Distribution of cells found with and without a mechanically-activated current (MA current) in both groups. Note the significant loss of mechanically-activated currents in OB-1 treated neurons. Fisher's exact test: vehicle vs. OB-1, P = 0.009; numbers indicate neurons recorded, data are displayed as percentage of neurons. (e) The mean of inactivation time constants (τ2) of mechanically-gated currents is shown; note that τ2 is slower in OB-1 treated neurons when compared to controls. Mann-Whitney U-test: vehicle vs. OB-1, P = 0.0056; numbers indicate individual recordings; data are displayed as individual τ2 and mean ± s.e.m and were recorded from DRG neurons in at least 9 independent experiments. ** P < 0.01.
Supplementary Figure 4 OB-1 has no effect upon ASIC3-mediated currents nor on stomatin inhibition of ASIC3.
(a) Neither the transient (T), nor sustained (S) phases of pH-evoked currents in CHO cells expressing ASIC3 were inhibited by OB-1 (20 μM). Student’s t-test: P = 0.2452, P = 0.7493; numbers indicate cells recorded (9 Vehicle, 8 OB-1), data are displayed as individual data points and mean peak current density ± s.e.m.. (b) Stomatin inhibits ASIC3-mediated pH gated currents, which is not modulated by OB-1. Student’s t-test, P = 0.4392, P = 0.8650; numbers indicate cells recorded (7 Vehicle, 7 OB-1); data are displayed as individual data points and mean peak current density ± s.e.m..
Supplementary Figure 5 Non silenced mechanoreceptors are functional after OB-1 treatment.
(a) The proportion of each class of mechanosensitive afferents recorded three hours after local OB-1 or vehicle application is displayed. After OB-1 treatment no significant differences were observed in the receptor proportions among Aβ-, Aδ- or C-fibers compared to vehicle treated skin. Fisher's exact test: Aβ-fibers OB-1 vs. vehicle, P = 0.1722; Aδ-fibers OB-1 vs. vehicle, P = 0.8199; C-fibers OB-1 vs. vehicle, P = 1.0000; numbers indicate single sensory fiber recordings derived from more than 10 independent experiments using adult mice; data are displayed as percentage of individual fibers. (b-e) Receptive field properties of single sensory afferents recorded from vehicle-treated or OB-1 treated skin are shown. (b-d) A series of ramp and hold stimuli with increasing velocities (0.075, 0.15, 0.45, 1.5 and 15 mm/s at 92 μm displacements) was applied to low threshold mechanoreceptors, i.e. slowly adapting mechanoreceptors (SAM) (b), rapidly adapting mechanoreceptors (RAM) (c) and D-hair receptors (d). Mean firing frequencies during the ramp phase are plotted as function of stimulus velocity; numbers indicate fibers recorded; data are displayed as mean number of action potentials ± s.e.m.. (e) An ascending series of displacement stimuli (32 – 1024 μm) using a constant stimulus velocity was applied to A-mechanonociceptors (A-M). Mean firing frequencies were plotted as function of displacement amplitudes showing no changes in mechanosenitivity in A-Ms in OB-1 treated compared to vehicle-treated skin. Two-way ANOVA: SAM vehicle vs. OB-1, P = 0.8924; RAM vehicle vs. OB-1, P = 0.565; D-hair vehicle vs. OB-1, P = 0.8437; A-M vehicle vs. OB-1, P = 0.0912; numbers indicate single sensory fiber recordings derived from more than 5 independent experiments using adult mice; data are displayed as mean number of action potentials ± s.e.m..
Supplementary Figure 6 Ultrastructure of the sciatic nerve after unilateral CCI.
(a,d) Schematic drawings of the section plane. (b,c,e,f) High magnification of electron micrograph images of the ipsilateral (b,e) or contralateral side (c,f) proximal and distal to the ligation. (b,c) Proximal to the injury normal myelinated and unmyelinated nerve fibers with typical ultrastructure and intact myelin sheaths or Remak bundles are seen. (e,f) Distal to the injury strong demyelination and axonal degeneration were observed only ipsilateral to the injury. Scale bar = 2 μm, SC spinal cord, SN sciatic nerve.
Supplementary Figure 7 OB-1 treatment in additional pain models.
(a) Development of tactile-evoked pain using the spared nerve injury (SNI) model. Paw withdrawal thresholds (PWTs) are displayed; note that wild type mice develop a prolonged tactile-evoked pain ipsilateral to the injury. Two-way ANOVA: SNI, ipsi vs. Sham, ipsi, P < 0.0001; numbers indicate adult mice treated (two cohorts); data are displayed as mean of individual median PWTs ± s.e.m.. (b) PWTs are displayed, showing no alleviation of tactile-evoked pain behavior after local OB-1 treatment. Mann-Whitney U-test: SNI vs. OB-1, P > 0.9999; numbers indicate adult mice treated (two cohorts); data are displayed as mean of individual median PWTs ± s.e.m.. (c) Stoml3 copy number derived from lumbar DRG L4 - L6 (two mice per preparation) determined using real-time PCR showing no up-regulation of Stoml3 mRNA after SNI. Mann-Whitney U-test: naïve, ipsi vs. SNI, ipsi, P > 0.9999; numbers indicate RNA preparations, data are displayed as mean copy number ± s.e.m.. (d-g) A single dose of NGF (1 mg/kg body weight) was injected i.p. into adult mice to induce hyperalgesia in wild type and Stoml3-/- mice. (d,e) PWT or paw withdrawal latencies (PWL) are displayed before and after NGF-induced hyperalgesia in wild type and Stoml3-/- mice showing that prominent symptoms of thermal and mechanical hyperalgesia were not different between the genotypes. (f,g) Both, PWTs and PWLs, were measured in response to OB-1 before and after systemic NGF-injection, note that OB-1 does not alleviate NGF-induced mechanical (g) or thermal (h) hyperalgesia. Two-way ANOVA: PWT WT vs. Stoml3-/- P = 0.6067 (g); PWL WT vs. Stoml3-/- (e) P = 0.1078. Mann-Whitney U-test: PWT vehicle paw naïve vs. OB-1 paw naive, P = 0.3615; PWL Vehicle paw naïve vs. OB-1 paw naïve, P = 0.699. Wilcoxon matched-pairs signed rank test: PWT syst. NGF vs. syst. NGF + OB-1 (f), P = 0.125; PWL syst. NGF vs. syst. NGF + OB-1, P = 0.1563; numbers indicate adult mice examined; data are displayed as mean of individual median PWTs (d,f) or PWLs (e,g); error bars indicate s.e.m.. *** P < 0.001 ns nonsignificant.
Supplementary Figure 8 Generation of the Stoml3lacZ and Stoml3StrepII mouse strains
(a) Schematic representation of the targeting vector, the wild type Stoml3 locus, and the mutated Stoml3lacZ allele, before and after removal of the self-excision neomycin (cre, neo) cassette. A 12 kb genomic region of Stoml3 locus containing exon 1 (E1, black), NLS-lacZ (blue), DTA (yellow), the self-excision neomycin cassette, loxP (red arrowhead), and SpeI (S) restriction sites are depicted. Green lines indicate the predicted fragment sizes obtained after SpeI digestion of genomic DNA. A green bar shows the 5’ sequence used as a probe for Southern blot analyses shown in b. Blue lines indicate the predicted fragment sizes obtained by genotyping the tail genomic DNA as shown in c. (b) Southern blot analysis of SpeI digested tail genomic DNA from Stoml3+/lacZ and wild type mice. (c) Genotyping analysis of tail genomic DNA from Stoml3+/lacZ, wild type, and Stoml3lacZ/lacZ mice. Stoml3-LacZ F and LacZ int R primers amplified a 649 bp fragment from the Stoml3lacZ mutant allele. Stoml3-LacZ F and Stoml3-LacZ R primers amplified a 875 bp fragment from the Stoml3 wild type allele, M: 100 bp ladder (Invitrogen). (d) Schematic representation of the targeting vector, the wild-type Stoml3 locus, and the mutated Stoml3StrepII allele, before and after removal of the neomycin (neo) cassette. A 12 kb genomic region of Stoml3 locus containing exon 1 (E1, black), Strep-TagII (red), DTA (yellow), the neomycin cassette (neo), loxP (blue arrowhead), and SpeI (S) restriction sites are depicted. Green lines indicate the predicted fragment sizes obtained after SpeI digestion of genomic DNA. A green bar shows the 5’ sequence used as a probe for Southern blot analyses shown in e. Blue lines indicate the predicted fragment sizes obtained by genotyping the tail genomic DNA as shown in f. (e) Southern blot analysis of SpeI digested tail genomic DNA from wild type, Stoml3+/StrepII and Stoml3StrepII/StrepII mice. (f) Genotyping analysis of tail genomic DNA from wild type, Stoml3+/StrepII, and Stoml3StrepII/StrepII mice. Stoml3-Strep F and Stoml3-Strep R2 primers amplified a 321 bp fragment from the Stoml3StrepII mutant allele. Stoml3-Strep F and Stoml3-Strep R1 primers amplified a 438 bp fragment from the Stoml3 wild type allele, M: 100 bp ladder (Invitrogen).
Supplementary Figure 9 Uncropped western blots.
Uncropped pictures of the Western blot shown in the manuscript in Figure 6. * unknown bands; LO: left-over of 55-70kDa bands shown in the top panel after stripping the blot.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1 and 2 (PDF 1726 kb)
Supplementary Dataset 1
Excel data sheet of results of in vitro screen for pharmacological activity of OB1 on a panel of 79 receptors. (XLS 47 kb)
Rights and permissions
About this article
Cite this article
Wetzel, C., Pifferi, S., Picci, C. et al. Small-molecule inhibition of STOML3 oligomerization reverses pathological mechanical hypersensitivity. Nat Neurosci 20, 209–218 (2017). https://doi.org/10.1038/nn.4454
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4454
This article is cited by
-
Sensory Schwann cells set perceptual thresholds for touch and selectively regulate mechanical nociception
Nature Communications (2024)
-
Expression pattern of Stomatin-domain proteins in the peripheral olfactory system
Scientific Reports (2022)
-
SPFH protein cage — one ring to rule them all
Cell Research (2022)
-
Stomatin modulates adipogenesis through the ERK pathway and regulates fatty acid uptake and lipid droplet growth
Nature Communications (2022)
-
USH2A is a Meissner’s corpuscle protein necessary for normal vibration sensing in mice and humans
Nature Neuroscience (2021)