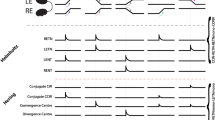
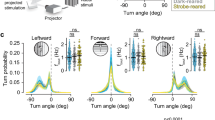
Abstract
Binocular competition is thought to drive eye-specific segregation in the developing visual system, potentially through Hebbian synaptic learning rules that are sensitive to correlations in afferent activity. Altering retinal activity can disrupt eye-specific segregation, but little is known about the temporal features of binocular activity that modulate visual map development. We used optogenetic techniques to directly manipulate retinal activity in vivo and identified a critical period before eye opening in mice when specific binocular features of retinal activity drive visual map development. Synchronous activation of both eyes disrupted segregation, whereas asynchronous stimulation enhanced segregation. The optogenetic stimulus applied was spatially homogenous; accordingly, retinotopy of ipsilateral projections was markedly perturbed, but contralateral retinotopy was unaffected or even improved. These results provide direct evidence that the synchrony and precise temporal pattern of binocular retinal activity during a critical period in development regulates eye-specific segregation and retinotopy in the developing visual system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Rakic, P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature 261, 467–471 (1976).
Shatz, C.J. The prenatal development of the cat's retinogeniculate pathway. J. Neurosci. 3, 482–499 (1983).
Feldheim, D.A. & O'Leary, D.D. Visual map development: bidirectional signaling, bifunctional guidance molecules, and competition. Cold Spring Harb. Perspect. Biol. 2, a001768 (2010).
Chapman, B. Necessity for afferent activity to maintain eye-specific segregation in ferret lateral geniculate nucleus. Science 287, 2479–2482 (2000).
Demas, J. et al. Failure to maintain eye-specific segregation in nob, a mutant with abnormally patterned retinal activity. Neuron 50, 247–259 (2006).
Koch, S.M. et al. Pathway-specific genetic attenuation of glutamate release alters select features of competition-based visual circuit refinement. Neuron 71, 235–242 (2011).
Huberman, A.D., Feller, M.B. & Chapman, B. Mechanisms underlying development of visual maps and receptive fields. Annu. Rev. Neurosci. 31, 479–509 (2008).
Hayakawa, I. & Kawasaki, H. Rearrangement of retinogeniculate projection patterns after eye-specific segregation in mice. PLoS ONE 5, e11001 (2010).
Stellwagen, D. & Shatz, C.J. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron 33, 357–367 (2002).
Mu, Y. & Poo, M.-m. Spike timing-dependent LTP/LTD mediates visual experience-dependent plasticity in a developing retinotectal system. Neuron 50, 115–125 (2006).
Butts, D.A., Kanold, P.O. & Shatz, C.J.A. Burst-based “Hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol. 5, e61 (2007).
Shah, R.D. & Crair, M.C. Retinocollicular synapse maturation and plasticity are regulated by correlated retinal waves. J. Neurosci. 28, 292–303 (2008).
Butts, D.A. & Kanold, P.O. The applicability of spike time dependent plasticity to development. Front. Synaptic Neurosci. 2, 30 (2010).
Meister, M., Wong, R.O., Baylor, D.A. & Shatz, C.J. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science 252, 939–943 (1991).
Moody, W.J. & Bosma, M.M. Ion channel development, spontaneous activity, and activity-dependent development in nerve and muscle cells. Physiol. Rev. 85, 883–941 (2005).
Stryker, M.P. & Strickland, S.L. Physiological segregation of ocular dominance columns depends on the pattern of afferent electrical activity. Invest. Ophthalmol. Vis. Sci. 25 (suppl.): 278 (1984).
Altmann, L., Luhmann, H.J., Greuel, J.M. & Singer, W. Functional and neuronal binocularity in kittens raised with rapidly alternating monocular occlusion. J. Neurophysiol. 58, 965–980 (1987).
Crair, M.C., Horton, J.C., Antonini, A. & Stryker, M.P. Emergence of ocular dominance columns in cat visual cortex by 2 weeks of age. J. Comp. Neurol. 430, 235–249 (2001).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Cardin, J.A. et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protoc. 5, 247–254 (2010).
Wang, H. et al. High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice. Proc. Natl. Acad. Sci. USA 104, 8143–8148 (2007).
Johnson, J. et al. Melanopsin-dependent light avoidance in neonatal mice. Proc. Natl. Acad. Sci. USA 107, 17374–17378 (2010).
Berson, D.M., Dunn, F.A. & Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073 (2002).
Thyagarajan, S. et al. Visual function in mice with photoreceptor degeneration and transgenic expression of Channelrhodopsin 2 in ganglion cells. J. Neurosci. 30, 8745–8758 (2010).
Badea, T.C., Cahill, H., Ecker, J., Hattar, S. & Nathans, J. Distinct roles of transcription factors Brn3a and Brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron 61, 852–864 (2009).
Ecker, J.L. et al. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron 67, 49–60 (2010).
Godement, P., Salaün, J. & Imbert, M. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J. Comp. Neurol. 230, 552–575 (1984).
Xu, H.-P. et al. An instructive role for patterned spontaneous retinal activity in mouse visual map development. Neuron 70, 1115–1127 (2011).
Butts, D.A. & Rokhsar, D.S. The information content of spontaneous retinal waves. J. Neurosci. 21, 961–973 (2001).
Chandrasekaran, A.R., Plas, D.T., Gonzalez, E. & Crair, M.C. Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse. J. Neurosci. 25, 6929–6938 (2005).
Muir-Robinson, G., Hwang, B.J. & Feller, M.B. Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J. Neurosci. 22, 5259–5264 (2002).
Pfeiffenberger, C., Yamada, J. & Feldheim, D.A. Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J. Neurosci. 26, 12873–12884 (2006).
Torborg, C.L., Hansen, K.A. & Feller, M.B. High frequency, synchronized bursting drives eye-specific segregation of retinogeniculate projections. Nat. Neurosci. 8, 72–78 (2005).
Tritsch, N.X., Yi, E., Gale, J.E., Glowatzki, E. & Bergles, D.E. The origin of spontaneous activity in the developing auditory system. Nature 450, 50–55 (2007).
Garaschuk, O., Linn, J., Eilers, J. & Konnerth, A. Large-scale oscillatory calcium waves in the immature cortex. Nat. Neurosci. 3, 452–459 (2000).
Watt, A.J. et al. Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat. Neurosci. 12, 463–473 (2009).
Kastanenka, K.V. & Landmesser, L.T. In vivo activation of Channelrhodopsin-2 reveals that normal patterns of spontaneous activity are required for motoneuron guidance and maintenance of guidance molecules. J. Neurosci. 30, 10575–10585 (2010).
Vislay-Meltzer, R.L., Kampff, A.R. & Engert, F. Spatiotemporal specificity of neuronal activity directs the modification of receptive fields in the developing retinotectal system. Neuron 50, 101–114 (2006).
Demas, J.A., Payne, H. & Cline, H.T. Vision drives correlated activity without patterned spontaneous activity in developing Xenopus retina. Dev. Neurobiol. 10.1002/dneu.20880 (2011).
Kerschensteiner, D. & Wong, R.O.L. A precisely timed asynchronous pattern of ON and OFF retinal ganglion cell activity during propagation of retinal waves. Neuron 58, 851–858 (2008).
Dan, Y. & Poo, M.-M. Spike timing-dependent plasticity: from synapse to perception. Physiol. Rev. 86, 1033–1048 (2006).
Weliky, M. & Katz, L.C. Disruption of orientation tuning visual cortex by artificially correlated neuronal activity. Nature 386, 680–685 (1997).
Xu, H.-P. et al. The immune protein CD3 is required for normal development of neural circuits in the retina. Neuron 65, 503–515 (2010).
Dhande, O.S. et al. Development of single retinofugal axon arbors in normal and Chrnb2 knock-out mice. J. Neurosci. 31, 3384–3399 (2011).
Huberman, A.D. et al. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron 59, 425–438 (2008).
Faguet, J., Maranhao, B., Smith, S.L. & Trachtenberg, J.T. Ipsilateral eye cortical maps are uniquely sensitive to binocular plasticity. J. Neurophysiol. 101, 855–861 (2009).
Petros, T.J., Rebsam, A. & Mason, C.A. Retinal axon growth at the optic chiasm: to cross or not to cross. Annu. Rev. Neurosci. 31, 295–315 (2008).
Crair, M.C., Gillespie, D.C. & Stryker, M.P. The role of visual experience in the development of columns in cat visual cortex. Science 279, 566–570 (1998).
Huberman, A.D., Speer, C.M. & Chapman, B. Spontaneous retinal activity mediates development of ocular dominance columns and binocular receptive fields in V1. Neuron 52, 247–254 (2006).
Swindell, E.C. et al. Rx-Cre, a tool for inactivation of gene expression in the developing retina. Genesis 44, 361–363 (2006).
Acknowledgements
We would like to thank members of the Crair laboratory for comments on the manuscript, J. Cardin and M. Higley for experimental guidance and Y. Zhang for technical help. This work was supported by US National Institutes of Health grants P30 EY000785, R01 EY015788 and R01 EY015788S to M.C.C. and by a Research to Prevent Blindness Challenge Grant to the Department of Ophthalmology & Visual Sciences. J.Z. was supported by a Brown-Coxe Fellowship. M.C.C. also thanks the family of W. Ziegler III for their support.
Author information
Authors and Affiliations
Contributions
J.Z. and M.C.C. designed the experiments. J.Z. conducted the stimulation experiments and analyzed the anatomical data. J.A. and J.Z. conducted the calcium imaging experiments and analyzed the data. H.-P.X. and J.Z. conducted the multielectrode array experiments and analyzed the data. J.Z. and M.C.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 434 kb)
Rights and permissions
About this article
Cite this article
Zhang, J., Ackman, J., Xu, HP. et al. Visual map development depends on the temporal pattern of binocular activity in mice. Nat Neurosci 15, 298–307 (2012). https://doi.org/10.1038/nn.3007
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3007
This article is cited by
-
Heterosynaptic plasticity-induced modulation of synapses
The Journal of Physiological Sciences (2023)
-
The Dorsal Nucleus of the Lateral Geniculate Body: Anatomy, Histology, Ontogenesis
Neuroscience and Behavioral Physiology (2023)
-
Mechanisms governing activity-dependent synaptic pruning in the developing mammalian CNS
Nature Reviews Neuroscience (2021)
-
Portrait of visual cortical circuits for generating neural oscillation dynamics
Cognitive Neurodynamics (2021)
-
Monocular enucleation alters retinal waves in the surviving eye
Neural Development (2018)