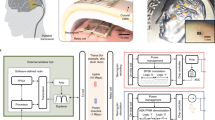
Abstract
Conventional neural recording systems restrict behavioral experiments to a flat indoor environment compatible with the cable that tethers the subject to recording instruments. To overcome these constraints, we developed a wireless multi-channel system for recording neural signals from rats. The device takes up to 64 voltage signals from implanted electrodes, samples each at 20 kHz, time-division multiplexes them into one signal and transmits that output by radio frequency to a receiver up to 60 m away. The system introduces <4 μV of electrode-referred noise, comparable to wired recording systems, and outperforms existing rodent telemetry systems in channel count, weight and transmission range. This allows effective recording of brain signals in freely behaving animals. We report measurements of neural population activity taken outdoors and in tunnels. Neural firing in the visual cortex was relatively sparse, correlated even across large distances and was strongly influenced by locomotor activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Wilson, M.A. & McNaughton, B.L. Dynamics of the hippocampal ensemble code for space. Science 261, 1055–1058 (1993).
Jog, M.S. et al. Tetrode technology: advances in implantable hardware, neuroimaging, and data analysis techniques. J. Neurosci. Methods 117, 141–152 (2002).
Felsen, G. & Dan, Y. A natural approach to studying vision. Nat. Neurosci. 8, 1643–1646 (2005).
Lewen, G.D., Bialek, W. & de Ruyter van Steveninck, R.R. Neural coding of naturalistic motion stimuli. Network 12, 317–329 (2001).
Reppas, J.B., Usrey, W.M. & Reid, R.C. Saccadic eye movements modulate visual responses in the lateral geniculate nucleus. Neuron 35, 961–974 (2002).
Barlow, H.B. in Sensory Communication (ed. Rosenblith, W.A.) 217–234 (MIT Press, Cambridge, Massachusetts, 1961).
Brun, V.H. et al. Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex. Hippocampus 18, 1200–1212 (2008).
Dabrowski, W. et al. Development of front-end ASICs for imaging neuronal activity in live tissue. Nucl. Instrum. Methods Phys. Res. A 541, 405–411 (2005).
Dabrowski, W., Grybos, P. & Litke, A.M. A low noise multichannel integrated circuit for recording neuronal signals using microelectrode arrays. Biosens. Bioelectron. 19, 749–761 (2004).
Grybos, P., Dabrowski, W., Hottowy, P., Fiutowski, T. & Bielewicz, B. Neuroplat64 – low noise CMOS integrated circuit for neural recording applications. Proc. 5th Int. Meet. Substrate-integrated Micro Electrode Arrays, 208–209 (2006).
Hottowy, P. et al. An MEA-based system for multichannel, low artifact stimulation and recording of neural activity. Proc. 6th Int. Meet. Substrate-integrated Micro Electrode Arrays, 261–265 (2008).
Pouzat, C., Mazor, O. & Laurent, G. Using noise signature to optimize spike-sorting and to assess neuronal classification quality. J. Neurosci. Methods 122, 43–57 (2002).
Uchida, N. & Mainen, Z.F. Speed and accuracy of olfactory discrimination in the rat. Nat. Neurosci. 6, 1224–1229 (2003).
Sinnamon, H.M. & Galer, B.S. Head movements elicited by electrical stimulation of the anteromedial cortex of the rat. Physiol. Behav. 33, 185–190 (1984).
Reid, R.C., Victor, J.D. & Shapley, R.M. The use of m-sequences in the analysis of visual neurons: linear receptive field properties. Vis. Neurosci. 14, 1015–1027 (1997).
Barthó, P. et al. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J. Neurophysiol. 92, 600–608 (2004).
McCormick, D.A., Connors, B.W., Lighthall, J.W. & Prince, D.A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J. Neurophysiol. 54, 782–806 (1985).
Niell, C.M. & Stryker, M.P. Highly selective receptive fields in mouse visual cortex. J. Neurosci. 28, 7520–7536 (2008).
Ji, D. & Wilson, M.A. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10, 100–107 (2007).
Sawinski, J. et al. Visually evoked activity in cortical cells imaged in freely moving animals. Proc. Natl. Acad. Sci. USA 106, 19557–19562 (2009).
Laughlin, S.B. & Sejnowski, T.J. Communication in neuronal networks. Science 301, 1870–1874 (2003).
Kohn, A., Zandvakili, A. & Smith, M.A. Correlations and brain states: from electrophysiology to functional imaging. Curr. Opin. Neurobiol. 19, 434–438 (2009).
Jermakowicz, W.J., Chen, X., Khaytin, I., Bonds, A.B. & Casagrande, V.A. Relationship between spontaneous and evoked spike-time correlations in primate visual cortex. J. Neurophysiol. 101, 2279–2289 (2009).
Gray, C.M., Maldonado, P.E., Wilson, M. & McNaughton, B. Tetrodes markedly improve the reliability and yield of multiple single-unit isolation from multi-unit recordings in cat striate cortex. J. Neurosci. Methods 63, 43–54 (1995).
Niell, C.M. & Stryker, M.P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010).
Calhoun, J.B. The Ecology and Sociology of the Norway Rat (U.S. Dept. of Health, Education, and Welfare, Public Health Service, Bethesda, Maryland, 1963).
Borton, D.A. et al. Wireless, high-bandwidth recordings from non-human primate motor cortex using a scalable 16-Ch implantable microsystem. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 5531–5534 (2009).
Harrison, R.R. et al. Wireless neural recording with single low-power integrated circuit. IEEE Trans. Neural Syst. Rehabil. Eng. 17, 322–329 (2009).
Gregory, J.A. et al. Low-cost wireless neural recording system and software. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 3833–3836 (2009).
Hampson, R.E., Collins, V. & Deadwyler, S.A. A wireless recording system that utilizes Bluetooth technology to transmit neural activity in freely moving animals. J. Neurosci. Methods 182, 195–204 (2009).
Yin, M., Lee, S.B. & Ghovanloo, M. In vivo testing of a low noise 32-channel wireless neural recording system. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 1608–1611 (2009).
Chestek, C.A. et al. HermesC: low-power wireless neural recording system for freely moving primates. IEEE Trans. Neural Syst. Rehabil. Eng. 17, 330–338 (2009).
Miranda, H. et al. A high-rate long-range wireless transmission system for simultaneous multichannel neural recording applications. IEEE Trans. Biomed. Circ. Syst. 4, 181–191 (2010).
Chalupa, L.M. & Willams, R.W. Eye, Retina, and Visual System of the Mouse (MIT Press, Cambridge, Massachusetts, 2008).
Yamamoto, J. & Wilson, M.A. Large-scale chronically implantable precision motorized microdrive array for freely behaving animals. J. Neurophysiol. 100, 2430–2440 (2008).
Lin, L. et al. Large-scale neural ensemble recording in the brains of freely behaving mice. J. Neurosci. Methods 155, 28–38 (2006).
Feierstein, C.E., Quirk, M.C., Uchida, N., Sosulski, D.L. & Mainen, Z.F. Representation of spatial goals in rat orbitofrontal cortex. Neuron 51, 495–507 (2006).
Siapas, A.G., Lubenov, E.V. & Wilson, M.A. Prefrontal phase locking to hippocampal theta oscillations. Neuron 46, 141–151 (2005).
Acknowledgements
We thank A. Leifer and E. Soucy for technical assistance and O. Mazor and A. Biewener for advice. Funding was provided by the McKnight Foundation (M.M., T.A.S.) the Gordon and Betty Moore Foundation (M.M.), the Polish Ministry of Science and Higher Education (W.D., P.H.), the National Science Foundation (PHY-0750525, A.M.L.) and the Burroughs Wellcome Fund Career Award at the Scientific Interface (A.S.).
Author information
Authors and Affiliations
Contributions
This manuscript was written by T.A.S. and M.M., with comments from all authors. The Neuroplat chip was designed by P.H., W.D. and A.M.L. The back and head boards were designed by A.M.L., V.F., S.K., A.S. and M.V.G. The wireless link was designed by T.A.S. and M.M. Implantations and experiments were performed by A.G.S. and E.V.L. (hippocampus), N.U. (frontal eye field), and T.A.S. and M.A. (V1). Analysis was performed by N.U. (FEF) and T.A.S. and M.M. (V1, hippocampus). M.M. and A.M.L. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 (PDF 136 kb)
Rights and permissions
About this article
Cite this article
Szuts, T., Fadeyev, V., Kachiguine, S. et al. A wireless multi-channel neural amplifier for freely moving animals. Nat Neurosci 14, 263–269 (2011). https://doi.org/10.1038/nn.2730
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2730
This article is cited by
-
Reduced slow-wave activity and autonomic dysfunction during sleep precede cognitive deficits in Alzheimer’s disease transgenic mice
Scientific Reports (2023)
-
Electro-Quasistatic Animal Body Communication for Untethered Rodent Biopotential Recording
Scientific Reports (2021)
-
Guide to preclinical models used to study the pathophysiology of idiopathic intracranial hypertension
Eye (2020)
-
Duets recorded in the wild reveal that interindividually coordinated motor control enables cooperative behavior
Nature Communications (2019)
-
A miniature multi-contrast microscope for functional imaging in freely behaving animals
Nature Communications (2019)