Abstract
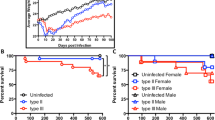
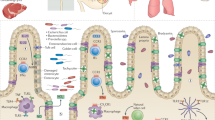
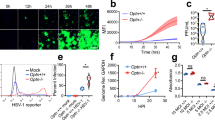
Globally, nearly 2 billion people are infected with the intracellular protozoan Toxoplasma gondii1. This persistent infection can cause severe disease in immunocompromised people and is epidemiologically linked to major mental illnesses2 and cognitive impairment3. There are currently no options for curing this infection. The lack of effective therapeutics is due partly to a poor understanding of the essential pathways that maintain long-term infection. Although it is known that Toxoplasma replicates slowly within intracellular cysts demarcated with a cyst wall, precisely how it sustains itself and remodels organelles in this niche is unknown. Here, we identify a key role for proteolysis within the parasite lysosomal organelle (the vacuolar compartment or VAC) in turnover of autophagosomes and persistence during neural infection. We found that disrupting a VAC-localized cysteine protease compromised VAC digestive function and markedly reduced chronic infection. Death of parasites lacking the VAC protease was preceded by accumulation of undigested autophagosomes in the parasite cytoplasm. These findings suggest an unanticipated function for parasite lysosomal degradation in chronic infection, and identify an intrinsic role for autophagy in the T. gondii parasite and its close relatives. This work also identifies a key element of Toxoplasma persistence and suggests that VAC proteolysis is a prospective target for pharmacological development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Montoya, J. G. & Liesenfeld, O. Toxoplasmosis. Lancet 363, 1965–1976 (2004).
Sutterland, A. L. et al. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta Psychiatr. Scand. 132, 161–179 (2015).
Bharti, A. R. et al. Latent toxoplasma infection and higher Toxoplasma gondii immunoglobulin G levels are associated with worse neurocognitive functioning in HIV-infected adults. Clin. Infect. Dis. 63, 1655–1660 (2016).
Parussini, F., Coppens, I., Shah, P. P., Diamond, S. L. & Carruthers, V. B. Cathepsin L occupies a vacuolar compartment and is a protein maturase within the endo/exocytic system of Toxoplasma gondii. Mol. Microbiol. 76, 1340–1357 (2010).
Miranda, K. et al. Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole. Mol. Microbiol. 76, 1358–1375 (2010).
Dou, Z., Coppens, I. & Carruthers, V. B. Non-canonical maturation of two papain-family proteases in Toxoplasma gondii. J. Biol. Chem. 288, 3523–3534 (2013).
Pittman, K. J., Aliota, M. T. & Knoll, L. J. Dual transcriptional profiling of mice and Toxoplasma gondii during acute and chronic infection. BMC Genomics 15, 806 (2014).
Fox, B. A. et al. Type II Toxoplasma gondii KU80 knockout strains enable functional analysis of genes required for cyst development and latent infection. Eukaryot. Cell 10, 1193–1206 (2011).
Dou, Z., McGovern, O., Di Cristina, M. & Carruthers, V. Toxoplasma gondii ingests and digests host cytosolic proteins. mBio 5, e01188–14 (2014).
Larson, E. T. et al. Toxoplasma gondii cathepsin L is the primary target of the invasion-inhibitory compound morpholinurea-leucyl-homophenyl-vinyl sulfone phenyl. J. Biol. Chem. 284, 26839–26850 (2009).
Parzych, K. R. & Klionsky, D. J. An overview of autophagy: morphology, mechanism, and regulation. Antioxid. Redox Signal. 20, 460–473 (2014).
Besteiro, S. Which roles for autophagy in Toxoplasma gondii and related apicomplexan parasites? Mol. Biochem. Parasitol. 184, 1–8 (2012).
Leveque, M. F. et al. Autophagy-related protein ATG8 has a noncanonical function for apicoplast inheritance in Toxoplasma gondii. mBio 6, e01446–15 (2015).
Tomlins, A. M. et al. Plasmodium falciparum ATG8 implicated in both autophagy and apicoplast formation. Autophagy 9, 1540–1552 (2013).
Besteiro, S., Brooks, C. F., Striepen, B. & Dubremetz, J. F. Autophagy protein Atg3 is essential for maintaining mitochondrial integrity and for normal intracellular development of Toxoplasma gondii tachyzoites. PLoS Pathog. 7, e1002416 (2011).
Ghosh, D., Walton, J. L., Roepe, P. D. & Sinai, A. P. Autophagy is a cell death mechanism in Toxoplasma gondii. Cell. Microbiol. 14, 589–607 (2012).
Barclay, J. et al. Role of the cysteine protease cathepsin S in neuropathic hyperalgesia. Pain 130, 225–234 (2007).
Di Cristina, M. et al. Transformed Toxoplasma gondii tachyzoites expressing the circumsporozoite protein of Plasmodium knowlesi elicit a specific immune response in rhesus monkeys. Infect. Immun. 67, 1677–1682 (1999).
Galizi, R. et al. Evidence of tRNA cleavage in apicomplexan parasites: half-tRNAs as new potential regulatory molecules of Toxoplasma gondii and Plasmodium berghei. Mol. Biochem. Parasitol. 188, 99–108 (2013).
Di Cristina, M. et al. Temporal and spatial distribution of Toxoplasma gondii differentiation into bradyzoites and tissue cyst formation in vivo. Infect. Immun. 76, 3491–3501 (2008).
Long, S., Wang, Q. & Sibley, L. D. Analysis of noncanonical calcium-dependent protein kinases in Toxoplasma gondii by targeted gene deletion using CRISPR/Cas9. Infect. Immun. 84, 1262–1273 (2016).
Lunghi, M., Galizi, R., Magini, A., Carruthers, V. B. & Di Cristina, M. Expression of the glycolytic enzymes enolase and lactate dehydrogenase during the early phase of Toxoplasma differentiation is regulated by an intron retention mechanism. Mol. Microbiol. 96, 1159–1175 (2015).
Di Cristina, M., Spaccapelo, R., Soldati, D., Bistoni, F. & Crisanti, A. Two conserved amino acid motifs mediate protein targeting to the micronemes of the apicomplexan parasite Toxoplasma gondii. Mol. Cell Biol. 20, 7332–7341 (2000).
Spano, F. et al. The SAG5 locus of Toxoplasma gondii encodes three novel proteins belonging to the SAG1 family of surface antigens. Int. J. Parasitol. 32, 121–131 (2002).
Dobrowolski, J. M. & Sibley, L. D. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84, 933–939 (1996).
Zhang, Y. W. et al. Disruption of the Toxoplasma gondii bradyzoite-specific gene BAG1 decreases in vivo cyst formation. Mol. Microbiol. 31, 691–701 (1999).
Coppens, I. & Joiner, K. A. Host but not parasite cholesterol controls Toxoplasma cell entry by modulating organelle discharge. Mol. Biol. Cell 14, 3804–3820 (2003).
Opperdoes, F. R. et al. Purification, morphometric analysis, and characterization of the glycosomes (microbodies) of the protozoan hemoflagellate Trypanosoma brucei. J. Cell Biol. 98, 1178–1184 (1984).
Reischl, U., Bretagne, S., Kruger, D., Ernault, P. & Costa, J. M. Comparison of two DNA targets for the diagnosis of toxoplasmosis by real-time PCR using fluorescence resonance energy transfer hybridization probes. BMC Infect. Dis. 3, 7 (2003).
Acknowledgements
This work was supported by National Institutes of Health grants R01AI120627 (to V.B.C. and M.D.C.), R01GM111703 (to M.B.) and R01AI060767 (to I.C.). Z.D. and A.J.S. received fellowship support from the American Heart Association. B.M.H. and G.K. were supported by National Institutes of Health training grant T32AI007528. O.L.M. was supported by a National Institutes of Health Ruth Kirschstein National Research Service Award F31AI118274. The authors thank S. Meshinchi and J. Whitfield at the University of Michigan for help with electron microscopy and cytokine analysis, respectively, as well as the Johns Hopkins University Microscopy facility. The authors thank L. Weiss, W. Sullivan Jr and L.D. Sibley for the provision of antibodies for this study.
Author information
Authors and Affiliations
Contributions
M.D.C., Z.D., M.L., O.L.M., G.K., M.-H.H., A.J.M., B.M.H., C.E. and V.B.C. designed the experiments. M.D.C., A.J.S., A.J.M., B.M.H. and V.B.C. wrote the manuscript. W.v.d.L. performed chemical synthesis. M.D.C., Z.D., M.L., O.L.M., G.K., M.H.-H., T.L.S., A.J.S., A.J.M., B.M.H. and I.C. performed the experiments. C.E., M.B. and S.B. provided advice or reagents that were essential for completion of the study. All authors discussed the findings and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–11 and Supplementary Table 1. (PDF 42826 kb)
Rights and permissions
About this article
Cite this article
Di Cristina, M., Dou, Z., Lunghi, M. et al. Toxoplasma depends on lysosomal consumption of autophagosomes for persistent infection. Nat Microbiol 2, 17096 (2017). https://doi.org/10.1038/nmicrobiol.2017.96
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2017.96
This article is cited by
-
In vitro maturation of Toxoplasma gondii bradyzoites in human myotubes and their metabolomic characterization
Nature Communications (2022)
-
Toxoplasma gondii metacaspase 2 is an important factor that influences bradyzoite formation in the Pru strain
Parasitology Research (2020)
-
A latent ability to persist: differentiation in Toxoplasma gondii
Cellular and Molecular Life Sciences (2018)
-
Toxoplasma-induced changes in host risk behaviour are independent of parasite-derived AaaH2 tyrosine hydroxylase
Scientific Reports (2017)