Abstract
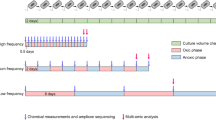
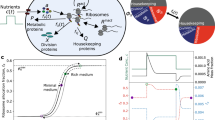
Most microorganisms live in environments where nutrients are limited and fluctuate over time. Cells respond to nutrient fluctuations by sensing and adapting their physiological state. Recent studies suggest phenotypic heterogeneity1 in isogenic populations as an alternative strategy in fluctuating environments, where a subpopulation of cells express a function that allows growth under conditions that might arise in the future2–9. It is unknown how environmental factors such as nutrient limitation shape phenotypic heterogeneity in metabolism and whether this allows cells to respond to nutrient fluctuations. Here, we show that substrate limitation increases phenotypic heterogeneity in metabolism, and this heterogeneity allows cells to cope with substrate fluctuations. We subjected the N2-fixing bacterium Klebsiella oxytoca to different levels of substrate limitation and substrate shifts, and obtained time-resolved single-cell measurements of metabolic activities using nanometre-scale secondary ion mass spectrometry (NanoSIMS). We found that the level of NH4+ limitation shapes phenotypic heterogeneity in N2 fixation. In turn, the N2 fixation rate of single cells during NH4+ limitation correlates positively with their growth rate after a shift to NH4+ depletion, experimentally demonstrating the benefit of heterogeneity. The results indicate that phenotypic heterogeneity is a general solution to two important ecological challenges—nutrient limitation and fluctuations—that many microorganisms face.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ackermann, M. A functional perspective on phenotypic heterogeneity in microorganisms. Nature Rev. Microbiol. 13, 497–508 (2015).
Acar, M., Mettetal, J. T. & van Oudenaarden, A. Stochastic switching as a survival strategy in fluctuating environments. Nature Genet. 40, 471–475 (2008).
Ratcliff, W. C. & Denison, R. F. Individual-level bet hedging in the bacterium Sinorhizobium meliloti. Curr. Biol. 20, 1740–1744 (2010).
New, A. M. et al. Different levels of catabolite repression optimize growth in stable and variable environments. PLoS Biol. 12, e1001764 (2014).
Kussell, E. & Leibler, S. Phenotypic diversity, population growth, and information in fluctuating environments. Science 309, 2075–2078 (2005).
Arnoldini, M., Mostowy, R., Bonhoeffer, S. & Ackermann, M. Evolution of stress response in the face of unreliable environmental signals. PLoS Comput. Biol. 8, e1002627 (2012).
Beaumont, H. J. E., Gallie, J., Kost, C., Ferguson, G. C. & Rainey, P. B. Experimental evolution of bet hedging. Nature 462, 90–93 (2009).
Arnoldini, M. et al. Bistable expression of virulence genes in salmonella leads to the formation of an antibiotic-tolerant subpopulation. PLoS Biol. 12, e1001928 (2014).
Balaban, N. Q., Merrin, J., Chait, R., Kowalik, L. & Leibler, S. Bacterial persistence as a phenotypic switch. Science 305, 1622–1625 (2004).
Dixon, R. & Kahn, D. Genetic regulation of biological nitrogen fixation. Nature Rev. Microbiol. 2, 621–631 (2004).
Pengra, R. M. & Wilson, P. W. Physiology of nitrogen fixation by Aerobacter aerogenes. J. Bacteriol. 75, 21–25 (1958).
Harpole, W. S. et al. Nutrient co-limitation of primary producer communities. Ecol. Lett. 14, 852–862 (2011).
Moore, C. M. et al. Processes and patterns of oceanic nutrient limitation. Nature Geosci. 6, 701–710 (2013).
Kleiner, D. Energy expenditure for cyclic retention of NH3/NH4+ during N2 fixation by Klebsiella pneumoniae. FEBS Lett. 187, 237–239 (1985).
So, L.-H. et al. General properties of transcriptional time series in Escherichia coli. Nature Genet. 43, 554–560 (2011).
Ozbudak, E. M., Thattai, M., Lim, H. N., Shraiman, B. I. & Van Oudenaarden, A. Multistability in the lactose utilization network of Escherichia coli. Nature 427, 737–740 (2004).
Kotte, O., Volkmer, B., Radzikowski, J. L. & Heinemann, M. Phenotypic bistability in Escherichia coli’s central carbon metabolism. Mol. Syst. Biol. 10, 736 (2014).
Solopova, A. et al. Bet-hedging during bacterial diauxic shift. Proc. Natl Acad. Sci. USA 111, 7427–7432 (2014).
Chubukov, V., Gerosa, L., Kochanowski, K. & Sauer, U. Coordination of microbial metabolism. Nature Rev. Microbiol. 12, 327–340 (2014).
Kochanowski, K. et al. Functioning of a metabolic flux sensor in Escherichia coli. Proc. Natl Acad. Sci. USA 110, 1130–1135 (2013).
Halm, H. et al. Co-occurrence of denitrification and nitrogen fixation in a meromictic lake, Lake Cadagno (Switzerland). Environ. Microbiol. 11, 1945–1958 (2009).
Musat, N. et al. A single-cell view on the ecophysiology of anaerobic phototrophic bacteria. Proc. Natl Acad. Sci. USA 105, 17861–17866 (2008).
Berry, D. et al. Host–compound foraging by intestinal microbiota revealed by single-cell stable isotope probing. Proc. Natl Acad. Sci. USA 110, 4720–4725 (2013).
Kopf, S. H. et al. Heavy water and 15N labeling with NanoSIMS analysis reveals growth-rate dependent metabolic heterogeneity in chemostats. Environ. Microbiol. 17, 2542–2556 (2015).
Zimmermann, M. et al. Phenotypic heterogeneity in metabolic traits among single cells of a rare bacterial species in its natural environment quantified with a combination of flow cell sorting and NanoSIMS. Front. Microbiol. 6, 243 (2015).
Hill, S. Influence of atmospheric oxygen concentration on acetylene reduction and efficiency of nitrogen fixation in intact Klebsiella pneumoniae. J. Gen. Microbiol. 93, 335–345 (1976).
Dabundo, R. et al. The contamination of commercial 15N2 gas stocks with 15N-labeled nitrate and ammonium and consequences for nitrogen fixation measurements. PLoS ONE 9, e110335 (2014).
Holmes, R. M., Aminot, A., Kerouel, R., Bethanie, H. A. & Peterson, B. J. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can. J. Fish. Aquat. Sci. 56, 1801–1808 (1999).
Polerecky, L. et al. Look@NanoSIMS—a tool for the analysis of nanoSIMS data in environmental microbiology. Environ. Microbiol. 14, 1009–1023 (2012).
Skinner, S. O., Sepúlveda, L. A., Xu, H. & Golding, I. Measuring mRNA copy number in individual Escherichia coli cells using single-molecule fluorescent in situ hybridization. Nature Protoc. 8, 1100–1113 (2013).
Arnold, W., Rump, A., Klipp, W., Priefer, U. B. & Pühler, A. Nucleotide sequence of a 24,206-base-pair DNA fragment carrying the entire nitrogen fixation gene cluster of Klebsiella pneumoniae. J. Mol. Biol. 203, 715–738 (1988).
Young, J. W. et al. Measuring single-cell gene expression dynamics in bacteria using fluorescence time-lapse microscopy. Nature Protoc. 7, 80–88 (2012).
Dixon, R., Kennedy, C., Kondorosi, A., Krishnapillai, V. & Merrick, M. Complementation analysis of Klebsiella pneumoniae mutants defective in nitrogen fixation. Mol. Gen. Genet. 157, 189–198 (1977).
Kaluza, K. & Hennecke, H. Regulation of nitrogenase messenger RNA synthesis and stability in Klebsiella pneumoniae. Arch. Microbiol. 130, 38–43 (1981).
Acknowledgements
The authors thank M. Zimmermann and T. Röösli for support during NanoSIMS and mRNA image segmentation, D. Franzke and D. Nini for support during NanoSIMS measurements, G. Klockgether and T. Max for IRMS measurements, and T. Egli, D.J. Kiviet, D.R. Johnson and H.-M. Fischer for discussions. The NanoSIMS instrument in the Laboratory for Biological Geochemistry was funded in part by the European Research Council Advanced Grant 246749 (BIOCARB) to A.M. This research was supported by a Leopoldina postdoctoral fellowship (LPDS 2009-42), a Marie-Curie Intra-European fellowship for career development (FP7-MC-IEF, 271929; Phenofix) and a Synthesis Grant of the ETH Zurich Center for Adaptation to a Changing Environment (ACE) to F.S., the Max Planck Society, ETH Zurich and Eawag.
Author information
Authors and Affiliations
Contributions
F.S., M.M.M.K. and M.A. designed the research. FS performed the experiments. S.L., S.E. and F.S. carried out the NanoSIMS measurements. S.L., G.L., S.E., A.M. and M.M.M.K. contributed analytical tools. F.S. and M.A. analysed data. F.S. and M.A. wrote the paper with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Sequence Information, Supplementary Figures 1-5, Table 1, Discussion and References. (PDF 537 kb)
Rights and permissions
About this article
Cite this article
Schreiber, F., Littmann, S., Lavik, G. et al. Phenotypic heterogeneity driven by nutrient limitation promotes growth in fluctuating environments. Nat Microbiol 1, 16055 (2016). https://doi.org/10.1038/nmicrobiol.2016.55
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2016.55
This article is cited by
-
Biofilm formation: mechanistic insights and therapeutic targets
Molecular Biomedicine (2023)
-
Single-cell view of deep-sea microbial activity and intracommunity heterogeneity
The ISME Journal (2023)
-
Single-cell isotope tracing reveals functional guilds of bacteria associated with the diatom Phaeodactylum tricornutum
Nature Communications (2023)
-
Spatial transcriptome uncovers rich coordination of metabolism in E. coli K12 biofilm
Nature Chemical Biology (2023)
-
Dynamic fluctuations in a bacterial metabolic network
Nature Communications (2023)