Abstract
We describe a lentivirus-encoded chimeric receptor, termed extracellular vesicle (EV)-internalizing receptor (EVIR), which enables the selective uptake of cancer-cell-derived EVs by dendritic cells (DCs). The EVIR enhances DC presentation of EV-associated tumor antigens to CD8+ T cells primarily through MHCI recycling and cross-dressing. EVIRs should facilitate exploring the mechanisms and implications of horizontal transfer of tumor antigens to antigen-presenting cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Simons, M. & Raposo, G. Curr. Opin. Cell Biol. 21, 575–581 (2009).
Tkach, M. & Théry, C. Cell 164, 1226–1232 (2016).
Becker, A. et al. Cancer Cell 30, 836–848 (2016).
Bobrie, A. & Théry, C. Biochem. Soc. Trans. 41, 263–267 (2013).
Wolfers, J. et al. Nat. Med. 7, 297–303 (2001).
Zhang, B., Yin, Y., Lai, R.C. & Lim, S.K. Front. Immunol. 5, 518 (2014).
Gu, X., Erb, U., Buchler, M.W. & Zöller, M. Int. J. Cancer 136, E74–E84 (2015).
Arteaga, C.L. et al. Nat. Rev. Clin. Oncol. 9, 16–32 (2011).
Amendola, M., Venneri, M.A., Biffi, A., Vigna, E. & Naldini, L. Nat. Biotechnol. 23, 108–116 (2005).
Squadrito, M.L. et al. Cell Rep. 8, 1432–1446 (2014).
Hogquist, K.A. et al. Cell 76, 17–27 (1994).
Dolan, B.P., Gibbs, K.D. Jr. & Ostrand-Rosenberg, S. J. Immunology 177, 6018–6024 (2006).
Wakim, L.M. & Bevan, M.J. Nature 471, 629–632 (2011).
Joffre, O.P., Segura, E., Savina, A. & Amigorena, S. Nat. Rev. Immunol. 12, 557–569 (2012).
Ludigs, K. et al. Nat. Commun. 7, 10554 (2016).
Ahmed, M. & Cheung, N.K. FEBS Lett. 588, 288–297 (2014).
Groux-Degroote, S., Guérardel, Y. & Delannoy, P. Chembiochem 18, 1146–1154 (2017).
Baer, C. et al. Nat. Cell Biol. 18, 790–802 (2016).
Palucka, A.K. & Coussens, L.M. Cell 164, 1233–1247 (2016).
Zhou, H. et al. J. Biol. Chem. 286, 31676–31683 (2011).
Squadrito, M.L. et al. Cell Rep. 1, 141–154 (2012).
Horwacik, I. et al. Mol. Cell. Proteomics 14, 2577–2590 (2015).
Goedhart, J. et al. Nat. Commun. 3, 751 (2012).
Leto, S.M. et al. Clin. Cancer Res. 21, 5519–5531 (2015).
Koller, B.H., Marrack, P., Kappler, J.W. & Smithies, O. Science 248, 1227–1230 (1990).
De Palma, M. & Naldini, L. Methods Enzymol. 346, 514–529 (2002).
Chopra, T. et al. Mol. Cell. Proteomics 13, 3014–3028 (2014).
Dunn, K.W., Kamocka, M.M. & McDonald, J.H. Am. J. Physiol. Cell Physiol. 300, C723–C742 (2011).
Acknowledgements
We are grateful to G. Guarda (University of Lausanne, UNIL, Switzerland) for providing BM cells of B2m-deficient mice; N. Haynes (Peter MacCallum Cancer Center, Melbourne, Australia) for MC38-OVA cells; A. Ribas (University of California, Los Angeles, California, USA) for SM1-OVA cells; A. Donda, J. Mach, and D. Speiser (UNIL) for valuable scientific advice; A. Lombardo (Vita-Salute San Raffaele University, Milan, Italy) for advice with CRISPR LVs; A. Royant (Institut de Biologie Structurale, Grenoble, France) for providing the mTq sequence; L. Trusolino (University of Torino Medical School, Italy) for providing the HER2 sequence; D. Thompson and L. Giesbrecht for technical help; and the EPFL core facilities of flow cytometry (FCCF), proteomics (PCF), and bioimaging/optics platform (BIOp), for skillful assistance. S.K.H. was supported by the Swiss Federal Commission for Scholarships for Foreign Students (Swiss Government Excellence Scholarship 2015.0430). This study was funded by grants from the European Research Council (ERC-CoG EVOLVE 725051), the EPFL Catalyze4Life program, and the Swiss National Foundation (SNF grant 31003A-165963) to M.D.P.
Author information
Authors and Affiliations
Contributions
M.L.S. constructed LVs, designed and performed research, analyzed and interpreted the data, assembled the display items, and wrote the manuscript. C.C. designed and analyzed EV internalization and MHCI recycling assays and contributed to the writing of the manuscript. S.K.H. performed some experiments. M.D.P. designed and supervised research, interpreted the data, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.L.S. and M.D.P. are inventors of a patent application filed by EPFL (WO2017134100A1), which describes the EVIR technology.
Integrated supplementary information
Supplementary Figure 1 Transduced monocytic cells stably express an anti-HER2 EVIR.
a) The top panel shows a schematic of the bidirectional lentiviral vector (LV) used to coordinately express the CtrlR or anti-HER2 EVIR, and GFP. The bottom panels illustrate representative flow cytometry dot plots showing expression of the EVIR, stained with an anti-F(ab')2 antibody, and GFP, in iBMM-CtrlR and iBMM-EVIR cells. The cells were analyzed 7 days post-transduction. One cell culture per LV type is shown; data are representative of 3 independent transduction experiments.
(b) Individual confocal images showing anti-scFv immunostaining (white), direct GFP fluorescence (green), actin fibers stained with phalloidin (magenta), and nuclear staining with DAPI (blue), in iBMM-CtrlR and anti-HER2 iBMM-EVIR cells. See Figure 1b for experimental details. Scale bar, 50 μm.
(c) Flow cytometry analysis of GFP expression in untransduced (UT) iBMMs, anti-HER2 iBMM-EVIR and iBMM-CtrlR cells. The iBMMs were passaged for 11 times during the 36 days that followed cell transduction. Data show percentage values. One cell culture per LV type is shown. The experiment was performed one time.
Numerical values for the experiments with quantitative data are presented in Supplementary Table 2.
Supplementary Figure 2 Anti-HER2 EVIR-expressing monocytic cells avidly adhere to HER2+ cancer cells.
(a) Flow cytometry histograms of HER2, mCh and mTq in MC38 cells transduced as indicated. One cell culture per LV type is shown; data are representative of at least 2 independent transduction experiments.
(b) Cell binding assay using Mo-EVIR/GFP (or control Mo-CtrlR/GFP) and MC38-HER2/mCh (or control MC38-mCh) cells incubated in suspension for 20 min at 1:1 ratio. The figure shows representative images of MC38 cells (mCh+, magenta) and monocytes (GFP+, green) transduced as indicated and imaged before flow cytometry; see Figure 1c for experimental details. Scale bar, 200 μm.
(c) Cell binding assay using Mo-EVIR/GFP (or control Mo-CtrlR/GFP) and MC38-HER2/mCh (or control MC38-mCh) cells incubated in suspension for 20 min at either 9:1 or 1:9 ratio. Data show the proportion of cells that appear either as single cells (green or pink, representing monocytes and MC38 cells, respectively) or in clusters (yellow, representing monocytes bound to MC38 cells), according to flow cytometry analysis. Data indicate mean values of two independent cell cultures per condition.
(d) Co-culture of iBMM-EVIR/GFP (or control iBMM-CtrlR/GFP), MC38-HER2/mTq (or control MC38-mTq), and iBMM-mCh cells under adherent conditions for 24 h before analysis. The left panel shows flow cytometry analysis of the percentage of events representing iBMMs either as single cells (green or pink) or in clusters with MC38 cells (yellow or grey); mean values of two independent cell cultures per condition are shown. The right panel shows representative flow cytometry dot plots. mTq/GFP double-positive events (clusters of iBMM-EVIR/GFP and MC38-HER2/mTq cells) fall in the doublet/aggregate area (red) based on FSC-A and FSC-H physical parameters.
Numerical values for the experiments with quantitative data are presented in Supplementary Table 2.
Supplementary Figure 3 DCs transduced with an anti-HER2 EVIR capture and internalize HER2+ EVs.
(a) Representative flow cytometry dot plots of GFP and EVIR (stained with an anti-dLNGFR antibody) expression in DC-CtrlR and DC-EVIR cells. One cell culture per LV type is shown; data are representative of 3 independent cell cultures.
(b) Representative flow cytometry histograms of HER2 expression in EV-mCh and EV-HER2/mCh. One EV preparation per type is shown; data are representative of 2 independent EV preparations.
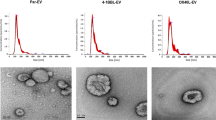
(c) Size distribution of EV-mCh and EV-HER2/mCh, determined by nanoparticle tracking analysis (NTA). Data show mean values ± SEM (n=3 technical measurements of one EV preparation per type). Data are representative of 3 independent EV preparations.
(d) Flow cytometry analysis of mCh in DC-EVIR either untreated or treated with EV-mCh or EV-HER2/mCh. Data are representative of 3 independent cell cultures per condition.
(e) Representative three-dimensional projections of confocal z-stack images showing nuclear DAPI (blue), anti-dLNGFR (green) and anti-mCh (magenta) immunostaining in DC-CtrlR and anti-HER2 DC-EVIR incubated for 2 h with MC38-HER2/mCh EVs. Scale bar, 10 μm. Images are representative of at least 30 cells per condition, analyzed from 3 independent experiments.
(f) Dose-response analysis of the median fluorescence intensity (MFI) of mCh in DC-CtrlR and DC-EVIR, either untreated or treated with EV-mCh or EV-HER2/mCh, and analyzed by flow cytometry. Data show mean values ± SEM (n=3 independent cell cultures per condition). Statistical analysis by two-way ANOVA with Sidak's multiple comparison test. The experiment was performed one time.
Numerical values for the experiments with quantitative data are presented in Supplementary Table 2.
Supplementary Figure 4 DCs transduced with an anti-HER2 EVIR show accelerated kinetics of EV and mCh internalization.
(a) Time course analysis of mCh localization in DC-EVIR or DC-CtrlR cells incubated with EV-HER2/mCh. The top panel shows representative confocal images of randomly selected dLNGFR+ cells, analyzed at the indicated time points after exposure to EVs. Data show anti-dLNGFR (green) and anti-mCh (magenta) immunostaining, and nuclear staining with DAPI (blue). Scale bar, 10 μm. The bottom panel shows analysis and quantification of the subcellular localization of the mCh signal in DC-CtrlR and DC-EVIR cells. Data show percentage values ± SEM (n=8 dLNGFR+ cells randomly selected per time point and condition). Results derived from individual cells, rather than by averaging different cell culture replicates, are reported in order to illustrate the variability of the parameter of interest (mCh localization in 128 independent cells from one experiment). Results are representative of 2 independent experiments. UT, untreated cells (not incubated with EVs).
(b) Flow cytometry analysis of mCh or mTq fluorescence in iBMM-EVIR cells, either untreated or treated with the indicated EV preparations. Data illustrate the MFI of mCh (top) and mTq (bottom), shown as mean values ± SEM (n=3 independent cell cultures per condition). Statistical analysis by one-way ANOVA with Tukey's multiple comparison test.
Numerical values for the experiments with quantitative data are presented in Supplementary Table 2.
Supplementary Figure 5 DC-EVIR cells promote T cell proliferation.
(a) Flow cytometry analysis of CD8+ OT-I proliferation, assessed by CellTrace dilution, analyzed after their co-culture with DC-CtrlR or DC-EVIR cells exposed to EV-HER2/OVA (top panels) or EV-OVA (bottom panels). Data show representative flow cytometry histograms of 3 independent cell cultures per condition (see Figure 1e for quantitative data).
(b) Flow cytometry analysis of CD8+ OT-I proliferation, assessed by CellTrace dilution, analyzed after treatment with EV-HER2/OVA in the absence of DCs, or after co-culture with DC-EVIR or DC-CtrlR cells and without EVs. Data show representative flow cytometry histograms of 3 independent cell cultures per condition.
(c) Dose-response of CD8+ OT-I cell proliferation, assessed by CellTrace dilution, after co-culture with DC-EVIR or DC-CtrlR in the presence of EV-HER2/OVA (left) or EV-OVA (right). Data show mean values ± SEM (n=3 independent cell cultures per condition). Statistical analysis by two-way ANOVA with Sidaks's multiple comparison test.
Numerical values for the experiments with quantitative data are presented in Supplementary Table 2.
Supplementary Figure 6 An anti-HER2 EVIR enhances EV-uptake by MHCI-deficient DCs.
(a) Schematic representation of the proviral CRISPR LV, which contains a control gRNA or a gRNA targeting H2kb or B2m, along with puromycin (PURO) resistance and inducible CAS9 transgenes.
(b) Flow cytometry dot plots showing H-2Kb or B2M expression in MC38 cells transduced with a LV without gRNA (control) or containing a gRNA either targeting H2kb or B2m. The analysis shows cell clones that have been selected for subsequent studies.
(c) PCR analysis of genomic OVA in the indicated MC38 cell lines. The analysis shows cell clones that have been selected for subsequent studies.
(d) Size distribution of H2kb- and B2m-deficient MC38-EVs, as determined by NTA. Data show mean values ± SEM (n=3 technical measurements of one EV preparation per type). Data are representative of 2 independent EV preparations.
(e) Flow cytometry showing the MFI of PKH67 in DC-CtrlR and DC-EVIR treated with PKH67-labeled, wild-type (WT, H2kb-proficient), H2kb- or B2m-deficient EVs that had been isolated from either MC38 or MC38-HER2 cells, as indicated. DCs were analyzed 24 h after exposure to EVs. Data show mean values ± SEM (n=3 independent cell cultures per condition); statistical analysis by two-way ANOVA with Sidak's multiple comparison test.
Numerical values for the experiments with quantitative data are presented in Supplementary Table 2.
Supplementary Figure 7 An anti-HER2 EVIR enhances EV uptake by DCs and cross-dressing with MHCI/antigen complexes.
(a) Flow cytometry analysis of CD8+ OT-I proliferation, assessed by CellTrace dilution, after co-culture with DC-CtrlR or anti-HER2 DC-EVIR cells, and EV-OVA (not containing HER2). The top panels show representative flow cytometry dot plots. The bottom panel shows the percentage of CD8+ OT-I cells that completed 1 to 3 (P1-3) or more than 3 (P>3) cell cycles. Data show mean percentage values ± SEM (n=3 independent cell cultures per condition).
(b, c) Flow cytometry dot plots showing B2M (b) and H-2Kb (c) expression in the indicated cell types. In (b), “B2m KO DC” refers to DCs isolated from the bone marrow of B2m KO mice (analysis performed once to verify a genetically-determined phenotype). In (c), “FVB DC” indicates DCs that were differentiated from the bone marrow of FVB/n mice (analysis performed once to verify a genetically-determined phenotype), whereas “Total CD45+ cells (NSG)” indicates all hematopoietic (CD45+) cells retrieved from a matrigel-embedded tumor containing H2kb KO MC38 cells and FVB/n DCs (analysis performed on 4 independent cell suspensions, of which one is shown). MHCI-competent bone marrow-derived DCs of C57Bl/6 mice (“DC”) and MC38 cancer cells (“MC38”) are shown for comparison (analysis performed once to verify a genetically-determined phenotype).
(d) Expression of H-2Kb in DCs transduced as indicated and exposed to the indicated EV preparations. Cells were analyzed 24 h after exposure to EVs. Data show mean values ± SEM (n=3 independent cell cultures per condition); statistical analysis by two-way ANOVA with Sidak's multiple comparison test.
Numerical values for the experiments with quantitative data are presented in Supplementary Table 2.
Supplementary Figure 8 An anti-HER2 EVIR promotes DC cross-dressing with MHCI/antigen complexes in a mouse tumor model.
(a-c) MC38 cancer cells (either H2kb wild-type or KO) and H2kb-deficient DCs (FVB/n origin) were co-mingled in matrigel plugs implanted in H2kb-deficient NSG mice (see Supplementary Figure 7c) and the tumors allowed to grow for 8 days. A schematic representation of the experiment is shown in (a). Panel (b) shows representative flow cytometry analyses of H-2Kb expression in the relevant cell types: dLNGFR+ cells, which represent transduced DC-EVIR or DC-CtrlR cells, and dLNGFR-negative CD45+ cells, which represent non-transduced hematopoietic cells recruited to the matrigel plug from the NSG host. Panel (c) shows analysis of the flow cytometry data. Data show mean values ± SEM (n=4 mice/condition) after normalizing the data to H-2Kb expression in the dLNGFR-negative cells (set to 1); statistical analysis by two-way ANOVA with Sidak's multiple comparison test.
(d) Representative flow cytometry dot plots showing the gating strategy employed in (a-c).
Numerical values for the experiments with quantitative data are presented in Supplementary Table 2.
Supplementary Figure 9 An anti-HER2 EVIR promotes EV internalization preferentially through macropinocytosis.
(a) The left panels show representative confocal images of DC-CtrlR and DC-EVIR after incubation with PKH67-labeled EV-HER2 (green) for 2 h, followed by washing with trypsin and acetate (or PBS), prior to fixation. The cells were stained with anti-dLNGFR antibodies (white) and their nuclei labeled with DAPI (blue). Scale bar, 10 μm. The right panel shows the quantification of the PKH67+ area; the total cell area was determined by dLNGFR+ membrane staining. Data show mean percentage values ± SEM (n=10 dLNGFR+ cells randomly selected per condition). Results derived from individual cells, rather than by averaging different cell culture replicates, are reported in order to illustrate the variability of the parameter of interest (PKH67+ area). Statistical analysis by two-way ANOVA with Sidak's multiple comparison test. Results are representative of 2 independent experiments.
(b) The left panels show representative confocal images of DC-CtrlR and DC-EVIR treated with the indicated endocytosis inhibitors, followed by incubation with PKH67-labeled EV-HER2 (green) for 2 h, prior to fixation. The cells were stained with anti-dLNGFR antibodies (not shown) and their nuclei labeled with DAPI (blue). Scale bar, 10 μm. The right panel shows the analysis of the PKH67+ area, calculated as in (a). Data show mean percentage values ± SEM (n=10 dLNGFR+ cells randomly selected per condition). For statistical analysis, see (a). In addition, one-way ANOVA analysis with Dunnett's multiple comparison test was performed, within either DC-CtrlR or DC-EVIR group, to compare each treatment condition with the corresponding untreated group. Results are representative of 2 independent experiments.
(c) The left panels show representative confocal images of DC-CtrlR and DC-EVIR exposed to PKH67-labelled EVs (green) along with dextran-TRITC (magenta) or transferrin-AF555 (magenta) for 2h, prior to fixation. The cells were stained with anti-dLNGFR antibodies (not shown) and their nuclei labeled with DAPI (blue). Scale bar, 10 μm. The right panel shows co-localization analysis of mCh with the indicated endocytosis marker, measured using the Manders' coefficient. Data show mean values ± SEM (n=10 dLNGFR+ cells randomly selected per condition). For statistical analysis, see (a). Results are representative of 2 independent experiments.
Numerical values for the experiments with quantitative data are presented in Supplementary Table 2.
Supplementary Figure 10 An anti-HER2 EVIR encourages EV sorting to recycling endosomes.
(a) Representative confocal images of DC-EVIR (left) and DC-CtrlR (right) incubated with EV-HER2/mCh (magenta) for 1 or 5 h and immunostained for the indicated endocytic markers (green). The cells were stained with anti-dLNGFR antibodies (not shown) and their nuclei labeled with DAPI (blue). Scale bar, 10 μm. Images are representative of 10 cells randomly selected per condition.
(b) Co-localization analysis of mCh and the indicated endocytosis marker, measured using the Manders' coefficient. Data show mean values ± SEM (n=10 cells randomly selected per condition). Results derived from individual cells, rather than by averaging different cell culture replicates, are reported in order to illustrate the variability of the parameter of interest (degree of co-localization). Statistical analysis by unpaired two-sided Student's t test. Results are representative of 2 independent experiments.
(c) Ratio of EV recycling (in RAB11+ endosomes) over degradation (in LAMP1+ lysosomes), calculated from the data in (b). Statistical analysis as in (b). Results are representative of 2 independent experiments.(d) Schematic representation of endocytic pathways relevant to the data shown in panels (a-c).
Numerical values for the experiments with quantitative data are presented in Supplementary Table 2.
Supplementary Figure 11 An anti-HER2 EVIR promotes MHCI recycling in DCs.
Representative confocal images of H-2Kb-negative DC-EVIR incubated with PKH67-labeled EV-HER2 (green), which were isolated from either H2kb-proficient (cells 1-4 and 6-9) or KO (cell 5) MC38 cells. Additional examples (including H2kb KO EVs assayed at 24 h) are shown in Figure 2c. The cells were analyzed 5 h (left panels) or 24 h (right panels) after exposure to the EVs. The cells were immunostained with antibodies against H-2Kb (magenta) and dLNGFR (white); nuclei are stained with DAPI (blue). Scale bar, 10 μm. Images are representative of 10 randomly selected cells per condition. Results are representative of 2 independent experiments.
Supplementary Figure 12 An anti-GD2 EVIR enhances EV uptake by DCs and presentation of a surrogate tumor antigen to T cells.
(a) Flow cytometry analysis of GD2 expression in MC38-mCh cells that had been transduced with the indicated GD2 synthase. The cells were analyzed once and employed in subsequent experiments.
(b) Flow cytometry analysis of mCh MFI in DC-CtrlR or anti-DG2 DC-EVIR untreated or treated with EV-mCh or EVs that had been isolated from GD2+ MC38-mCh cells (EV-GD2/mCh). Data show mean MFI values ± SEM (n=3 independent cell cultures per condition); statistical analysis by two-way ANOVA with Sidak's multiple comparison test.
(c) Flow cytometry analysis of GD2 expression in SM1-OVA cells that had been transduced with the indicated GD2 synthase. The cells were analyzed once and employed in subsequent experiments.
(d) Flow cytometry analysis of H-2Kb expression in SM1-GD2/OVA cells, untreated or treated with recombinant IFNγ as indicated. The experiment was performed once.
(e) Size distribution of EV-SM1-OVA, either with or without GD2, as determined by NTA. Data show mean values ± SEM (n=3 technical measurements of one EV preparation per type). Data are representative of 2 independent EV preparations.
(f) Flow cytometry analysis of CD8+ OT-I cell proliferation assessed by CellTrace dilution after co-culture with the indicated DCs and EVs. The left panels show representative flow cytometry dot plots (one cell culture of 3 performed per condition). The right panel shows the percentage of CD8+ OT-I cells that completed at least 1 cell cycle. Data show mean percentages ± SEM (n=3 independent cell cultures per condition); statistical analysis by two-way ANOVA with Sidak's multiple comparison test.
Numerical values for the experiments with quantitative data are presented in Supplementary Table 2.
Supplementary Figure 13 Analysis of splenic T cells in tumor-bearing mice.
Representative flow cytometry dot plots showing the gating strategy employed to identify dextramer+ CD8+ T cells in the spleens of MC38-bearing mice (see Figure 2e).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 (PDF 2348 kb)
Supplementary Table 1
Mass-spectrometry data (XLSX 960 kb)
Supplementary Table 2
Source data (XLSX 64 kb)
Supplementary Table 3
List of antibodies (XLSX 42 kb)
Rights and permissions
About this article
Cite this article
Squadrito, M., Cianciaruso, C., Hansen, S. et al. EVIR: chimeric receptors that enhance dendritic cell cross-dressing with tumor antigens. Nat Methods 15, 183–186 (2018). https://doi.org/10.1038/nmeth.4579
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4579
This article is cited by
-
An autologous antigen-agnostic dendritic cell therapy that forgoes antigen loading
Nature Cancer (2024)
-
Roles of cancer-associated fibroblasts (CAFs) in anti- PD-1/PD-L1 immunotherapy for solid cancers
Molecular Cancer (2023)
-
Cytokine-armed dendritic cell progenitors for antigen-agnostic cancer immunotherapy
Nature Cancer (2023)
-
Engineering of Trophoblast Extracellular Vesicle-Delivering Hydrogels for Localized Tolerance Induction in Cell Transplantation
Cellular and Molecular Bioengineering (2023)
-
Reversing insufficient photothermal therapy-induced tumor relapse and metastasis by regulating cancer-associated fibroblasts
Nature Communications (2022)