Abstract
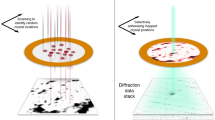
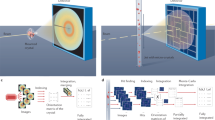
We report a method for serial X-ray crystallography at X-ray free-electron lasers (XFELs), which allows for full use of the current 120-Hz repetition rate of the Linear Coherent Light Source (LCLS). Using a micropatterned silicon chip in combination with the high-speed Roadrunner goniometer for sample delivery, we were able to determine the crystal structures of the picornavirus bovine enterovirus 2 (BEV2) and the cytoplasmic polyhedrosis virus type 18 polyhedrin, with total data collection times of less than 14 and 10 min, respectively. Our method requires only micrograms of sample and should therefore broaden the applicability of serial femtosecond crystallography to challenging projects for which only limited sample amounts are available. By synchronizing the sample exchange to the XFEL repetition rate, our method allows for most efficient use of the limited beam time available at XFELs and should enable a substantial increase in sample throughput at these facilities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zocher, G. et al. A sialic acid binding site in a human picornavirus. PLoS Pathog. 10, e1004401 (2014).
Fry, E.E., Abrescia, N.G.A. & Stuart, D.I. in Macromolecular Crystallography: Conventional and High-Throughput Methods (eds. Sanderson, M.R. & Skelly, J.V.), 245–264 (Oxford, 2007).
Fry, E.E., Grimes, J. & Stuart, D.I. Virus crystallography. Mol. Biotechnol. 12, 13–23 (1999).
Axford, D. et al. In situ macromolecular crystallography using microbeams. Acta Crystallogr. D Biol. Crystallogr. 68, 592–600 (2012).
Hope, H. Cryocrystallography of biological macromolecules: a generally applicable method. Acta Crystallogr. B 44, 22–26 (1988).
Owen, R.L., Rudiño-Piñera, E. & Garman, E.F. Experimental determination of the radiation dose limit for cryocooled protein crystals. Proc. Natl. Acad. Sci. USA 103, 4912–4917 (2006).
Meents, A., Gutmann, S., Wagner, A. & Schulze-Briese, C. Origin and temperature dependence of radiation damage in biological samples at cryogenic temperatures. Proc. Natl. Acad. Sci. USA 107, 1094–1099 (2010).
Chapman, H.N. et al. Femtosecond X-ray protein nanocrystallography. Nature 470, 73–77 (2011).
Boutet, S. et al. High-resolution protein structure determination by serial femtosecond crystallography. Science 337, 362–364 (2012).
Redecke, L. et al. Natively inhibited Trypanosoma brucei cathepsin B structure determined by using an X-ray laser. Science 339, 227–230 (2013).
Ayyer, K. et al. Macromolecular diffractive imaging using imperfect crystals. Nature 530, 202–206 (2016).
Neutze, R., Wouts, R., van der Spoel, D., Weckert, E. & Hajdu, J. Potential for biomolecular imaging with femtosecond X-ray pulses. Nature 406, 752–757 (2000).
Barty, A. et al. Self-terminating diffraction gates femtosecond X-ray nanocrystallography measurements. Nat. Photonics 6, 35–40 (2012).
Kupitz, C. et al. Serial time-resolved crystallography of photosystem II using a femtosecond X-ray laser. Nature 513, 261–265 (2014).
Tenboer, J. et al. Time-resolved serial crystallography captures high-resolution intermediates of photoactive yellow protein. Science 346, 1242–1246 (2014).
Perera, R., Khaliq, M. & Kuhn, R.J. Closing the door on flaviviruses: entry as a target for antiviral drug design. Antiviral Res. 80, 11–22 (2008).
Connolly, S.A., Jackson, J.O., Jardetzky, T.S. & Longnecker, R. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat. Rev. Microbiol. 9, 369–381 (2011).
Harrison, J.S. et al. Role of electrostatic repulsion in controlling pH-dependent conformational changes of viral fusion proteins. Structure 21, 1085–1096 (2013).
Weierstall, U. et al. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography. Nat. Commun. 5, 3309 (2014).
DePonte, D.P. et al. Gas dynamic virtual nozzle for generation of microscopic droplet streams. J. Phys. D Appl. Phys. 41, 195505 (2008).
Caffrey, M. & Cherezov, V. Crystallizing membrane proteins using lipidic mesophases. Nat. Protoc. 4, 706–731 (2009).
Liu, W. et al. Serial femtosecond crystallography of G protein-coupled receptors. Science 342, 1521–1524 (2013).
Fromme, R. et al. Serial femtosecond crystallography of soluble proteins in lipidic cubic phase. IUCrJ 2, 545–551 (2015).
Sugahara, M. et al. Grease matrix as a versatile carrier of proteins for serial crystallography. Nat. Methods 12, 61–63 (2015).
Sugahara, M. et al. Oil-free hyaluronic acid matrix for serial femtosecond crystallography. Sci. Rep. 6, 24484 (2016).
Conrad, C.E. et al. A novel inert crystal delivery medium for serial femtosecond crystallography. IUCrJ 2, 421–430 (2015).
Lawrence, R.M. et al. Serial femtosecond X-ray diffraction of enveloped virus microcrystals. Struct. Dyn. 2, 041720 (2015).
Zarrine-Afsar, A. et al. Crystallography on a chip. Acta Crystallogr. D Biol. Crystallogr. 68, 321–323 (2012).
Hunter, M.S. et al. Fixed-target protein serial microcrystallography with an x-ray free electron laser. Sci. Rep. 4, 6026 (2014).
Mueller, C. et al. Fixed target matrix for femtosecond time-resolved and in situ serial micro-crystallography. Struct. Dyn. 2, 054302 (2015).
Roedig, P. et al. A micro-patterned silicon chip as sample holder for macromolecular crystallography experiments with minimal background scattering. Sci. Rep. 5, 10451 (2015).
Roedig, P. et al. Room-temperature macromolecular crystallography using a micro-patterned silicon chip with minimal background scattering. J. Appl. Cryst. 49, 968–975 (2016).
Oghbaey, S. et al. Fixed target combined with spectral mapping: approaching 100% hit rates for serial crystallography. Acta Crystallogr. D Struct. Biol. 72, 944–955 (2016).
Cohen, A.E. et al. Goniometer-based femtosecond crystallography with X-ray free electron lasers. Proc. Natl. Acad. Sci. USA 111, 17122–17127 (2014).
Murray, T.D. et al. A high-transparency, micro-patternable chip for X-ray diffraction analysis of microcrystals under native growth conditions. Acta Crystallogr. D Biol. Crystallogr. 71, 1987–1997 (2015).
Baxter, E.L. et al. High-density grids for efficient data collection from multiple crystals. Acta Crystallogr. D Struct. Biol. 72, 2–11 (2016).
Owen, R.L. et al. Low-dose fixed-target serial synchrotron crystallography. Acta Crystallogr. D Struct. Biol. 73, 373–378 (2017).
Sherrell, D.A. et al. A modular and compact portable mini-endstation for high-precision, high-speed fixed target serial crystallography at FEL and synchrotron sources. J. Synchrotron Radiat. 22, 1372–1378 (2015).
Smyth, M. et al. Preliminary crystallographic analysis of bovine enterovirus. J. Mol. Biol. 231, 930–932 (1993).
Smyth, M. et al. Implications for viral uncoating from the structure of bovine enterovirus. Nat. Struct. Biol. 2, 224–231 (1995).
Goens, S.D., Botero, S., Zemla, A., Zhou, C.E. & Perdue, M.L. Bovine enterovirus 2: complete genomic sequence and molecular modelling of a reference strain and a wild-type isolate from endemically infected US cattle. J. Gen. Virol. 85, 3195–3203 (2004).
Filman, D.J. et al. Structural factors that control conformational transitions and serotype specificity in type 3 poliovirus. EMBO J. 8, 1567–1579 (1989).
Coulibaly, F. et al. The molecular organization of cypovirus polyhedra. Nature 446, 97–101 (2007).
Ji, X. et al. How baculovirus polyhedra fit square pegs into round holes to robustly package viruses. EMBO J. 29, 505–514 (2010).
Anduleit, K. et al. Crystal lattice as biological phenotype for insect viruses. Protein Sci. 14, 2741–2743 (2005).
Ji, X. et al. Polyhedra structures and the evolution of the insect viruses. J. Struct. Biol. 192, 88–99 (2015).
Ginn, H.M. et al. Structure of CPV17 polyhedrin determined by the improved analysis of serial femtosecond crystallographic data. Nat. Commun. 6, 6435 (2015).
Sanchez-Weatherby, J. et al. Improving diffraction by humidity control: a novel device compatible with X-ray beamlines. Acta Crystallogr. D Biol. Crystallogr. 65, 1237–1246 (2009).
Axford, D., Ji, X., Stuart, D.I. & Sutton, G. In cellulo structure determination of a novel cypovirus polyhedrin. Acta Crystallogr. D Biol. Crystallogr. 70, 1435–1441 (2014).
Roedig, P. et al. Sample preparation and data collection for high-speed fixed-target serial femtosecond crystallography. Protocol Exchange http://dx.doi/org/10.1038/protex.2017.059 (2017).
Walter, T.S. et al. A procedure for setting up high-throughput nanolitre crystallization experiments: crystallization workflow for initial screening, automated storage, imaging and optimization. Acta Crystallogr. D Biol. Crystallogr. 61, 651–657 (2005).
Wheeler, M.J., Russi, S., Bowler, M.G. & Bowler, M.W. Measurement of the equilibrium relative humidity for common precipitant concentrations: facilitating controlled dehydration experiments. Acta Crystallogr. F Struct. Biol. Cryst. Commun. 68, 111–114 (2012).
Ginn, H.M., Evans, G., Sauter, N.K. & Stuart, D.I. On the release of cppxfel for processing X-ray free-electron laser images. J. Appl. Crystallogr. 49, 1065–1072 (2016).
Ginn, H.M. et al. TakeTwo: an indexing algorithm suited to still images with known crystal parameters. Acta Crystallogr. D Struct. Biol. 72, 956–965 (2016).
Brunger, A.T. Version 1.2 of the Crystallography and NMR system. Nat. Protoc. 2, 2728–2733 (2007).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Acknowledgements
We thank the LCLS staff for their support during experiment LH90. Use of the LCLS, SLAC National Accelerator Laboratory, is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-76SF00515. This work was further supported by the European Cluster of Advanced Laser Light Sources (EUCALL) and the Virtual Institute VH-VI-403 of the Helmholtz Association. H.M.G. was supported by the Wellcome Trust (studentship 075491/04). D.I.S. was supported by the Medical Research Council, grant MR/N00065X/1 and previously by G1000099. N.K.S. was supported by the US National Institutes of Health grants GM102520 and GM117126 for data processing methods. We further thank J. Bergtholdt from DESY for preparation of the technical drawings of the Roadrunner setup.
Author information
Authors and Affiliations
Contributions
P.R., T.P., P.F., J.M., A.W., and A.M. designed the experiment. P.R., G.S., K.H., T.S.W., R.D., I.V., M.W., A.K., J.K., and D.J.R. were involved in sample preparation. P.R., T.P., G.S., K.H., J.M., P.F., R.D., B.R., M.S., S.N., D.S.D., R.A.-M., C.D., A.W., and A.M. participated in data collection. P.R., H.M.G., A.S.B., I.D.Y., T.M.-C., N.K.S., J.R., E.E.F., and D.I.S. analyzed the data. P.R., H.M.G., D.I.S., A.W., and A.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.M. is a CEO and shareholder of the DESY spin-off company Suna-Precision GmbH. T.P. and B.R are also shareholders. Suna-Precision sells technical equipment for experiments with X-rays, including different microstructured silicon chips for serial crystallography experiments. I.V. is shareholder and CTO of the company FinnLitho, which produced the silicon chips used in the experiment.
Integrated supplementary information
Supplementary Figure 1 Technical overview drawing of the Roadrunner goniometer.
The goniometer consists of three main components: an inline sample-viewing microscope, a high-precision scanning unit for fast scanning, and a motorized beam pipe unit. All components are mounted on a common support frame to achieve high mechanical stability. The overall dimensions of the support frame are 350 mm along the beam direction, the height is 515 mm, and the width is 400 mm. The beam and sample height is 250 mm above the base plate. The minimum possible distance between sample and detector surface is 50 mm allowing for the collection of high-resolution diffraction data. For better visibility, the open flow cryostat for measurements at cryogenic temperatures and the nozzle releasing a humidified gas stream for room-temperature measurements are not shown.
Supplementary Figure 2 Technical drawing of the scanning unit of the Roadrunner goniometer.
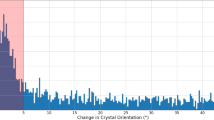
As illustrated in Supplementary Figure 1, the scanning unit is mounted vertically and hanging from the outer support frame. Upper element of the scanning unit is a Kohzu RA10A-W rotation stage. The rotation axis allows a +/- 60° rotation of the whole scanning unit along the vertical axis. The stage is used for rotational alignment of the chip along the vertical axis and can be further used for rotation of the chip to avoid obtaining incomplete diffraction datasets due to preferred orientation of the crystals on the chip. A stepper motor driven Kohzu XA07-RA translation stage is mounted below the rotation axis, which allows adjustment of the chip along a horizontal axis. This axis is used to position the chip surface in the center of rotation of the vertical rotation axis. The main high-precision scanning unit is mounted below the horizontal translation stage. It consists of two piezo-motor driven translation stages, which are equipped with incremental encoders and allow a fast translation of the chip with a speed of up to 2.5 mm/s. The achievable resolution of the setup is 100 nm. In case even higher resolution is required the scanning stages can be additionally equipped with interferometric sensors. For fast chip scanning synchronized to the arrival of the LCLS pulses only the horizontal axis is used, as this movement is not affected by gravity. The vertical axis is only used for small corrections e.g. arising from a not exact horizontal orientation of the hole pattern. The chip is mounted onto the scanning unit using a magnetic mount. A clamp attached to the magnetic mount is used to ensure the pre-orientation of the chips by allowing mounting of the chip in one angular position only.
Supplementary Figure 3 Technical drawing of the very compact Roadrunner inline sample-viewing microscope.
Main component is an Olympus LMPLFLN50X light microscope objective with 50x magnification and a numerical aperture of NA=0.5 with a 0.5 mm diameter hole through its center for the X-rays to pass through. The X-ray beam path is indicated in red. To reduce scattering background a 0.5 mm outer diameter and 0.35 mm inner diameter molybdenum tube is inserted into the drill hole extending to about 3 mm before the sample position. The optical light path is indicated in green. After leaving the microscope the light path is deflected by 90 degrees using a prism mirror, which is also equipped with a drill hole to pass the X-rays. After exiting the tube lens (f=80 mm), the magnified image of the sample is recorded using a ProSilica GC1360 Gigabit Ethernet CCD camera. The spatial resolution of the microscope setup is better than 1 μm.
Supplementary Figure 4 Technical drawing of the postsample beam pipe unit of the Roadrunner goniometer.
The beam pipe consists of a molybdenum tube with an inner diameter (ID) of 0.35 mm and an outer diameter (OD) of 0.5 mm. The upstream end of the pipe is placed about 15 mm behind the sample. The far end of the smaller diameter tube is inserted into a second larger tube with an ID of 0.55 mm and an OD of 1 mm, which extends several tens of millimeters downstream into the central hole of the CSPAD detector. The larger tube is manually pre-aligned in the direction of the direct beam path. It is mounted on a manually operated translation stage for adjustment along the beam direction. Two stepper motor operated translation stages allow fine adjustment of the beam pipe perpendicular to the X-ray beam and to guarantee that the primary beam is well enclosed by the beam pipe. By enclosing the direct beam shortly behind the sample in the molybdenum tube all X-rays scattered by air on the way down to the detector are absorbed in the beam pipe walls and therefore do not contribute to background scattering onto the detector. This results in a significant reduction of the background level from air scattering.
Supplementary Figure 5 Picture of the Roadrunner goniometer installed at the XPP instrument at LCLS for measurements at cryogenic temperatures.
The beam first passes through a drill hole in the inline sample viewing microscope used to visualize and align the sample. A molybdenum collimator tube inserted into the drill hole in the objective lens absorbs radiation caused by air scattering in order to reduce background scattering. The silicon chip sample holder is mounted from the top on the high-precision goniometer stage (not shown). The sample is cooled to temperatures down to 80 K by a cold nitrogen gas flow. Behind the sample the direct beam enters a molybdenum beam pipe absorbing X-rays scattered by air. Background scattering is further reduced by generating a helium gas sheet along the primary beam path between the sample and the beam pipe enclosing the direct beam behind the sample. For room-temperature measurements the cold nitrogen gas flow is replaced by a stream of humidified gas to prevent dehydration of the crystals (not shown here).
Supplementary Figure 6 Graphical user interface of the Roadrunner goniometer.
On the left side of the graphical user interface the image of the inline sample-viewing microscope is displayed – showing a chip mounted on the goniometer. The distance between the pores of the chip shown is 10 μm and the pore diameter ~5 μm. The red dot in the center of the image represents the X-ray beam position. The green and yellow lines are support lines to define the scan grid. Below the microscope image and on the right side of the GUI several control parameters and motor positions are displayed and can be adjusted.
Supplementary Figure 7 Synchronization scheme of the fast scanning axis with the timing signal of LCLS.
Once the goniometer is in the right position to perform a line scan a TTL “scanner ready” signal is sent from the goniometer controller to the LCLS control system. When the signal is received by the LCLS control systems it starts sending a predefined sequence of TTL signals co-incident to the X-ray pulse arrival times with a frequency of 120 Hz. At the beginning the pulse picker at XPP is blocking the beam and no X-ray pulses are delivered to the sample. These first timing pulses are used for acceleration of the sample scanner and to synchronize the position of the pores with the beam position and arrival time of the X-ray pulses. Once the pore position is in phase with the arrival of the X-ray pulses the pulse picker opens and X-rays are hitting the crystals located in the pores of the chip and diffraction images are recorded. Once the end of a line is reached, the pulse picker closes and the X-rays are blocked. A few more timing signals are sent out for deceleration of the chip. After no signals are received by the goniometer controller anymore, the goniometer moves to the starting position of the next scan line, sends out the “scanner ready” signal to the controller, which then continues scanning the line in reverse direction and so on. After the number of predefined lines are scanned in a meander-like fashion no “scanner ready’ signal is sent out anymore and data collection is finished.
Supplementary Figure 8 BEV2 phase extension of XFEL data.
(a) Stereo diagram of a 5-fold real space averaged electron density map at 5 Å resolution prior to phase extension. (b) Electron density map after phase extension to 2.5 Å by cyclic real space averaging and solvent flattening. (c) Scatter plot of phases derived from XFEL data via phase extension and those derived from an averaged map calculated from synchrotron data. Phases are only shown for reflections in the resolution range 5 Å to 2.5 Å.
Supplementary Figure 9 BEV2 structure determination on the basis of XFEL data by molecular replacement using FMDV A22 as a starting model.
(a) Stereo diagram showing the structural differences between BEV2 and FMDV A22 protomers. VP1, VP2, VP3 and VP4 of BEV2 are shown in blue, green, red and yellow respectively, FMDV A22 in grey. (b & c) Stereo diagram showing the electron density maps for the north wall of the canyon which surrounds the icosahedral 5-fold axes (note that FMDVs do not have such a canyon); (b) shows 5-fold real space averaged maps, (c) shows the map after cyclic real space averaging and solvent flattening. The initial map was calculated using phases derived from the FMDV A22 model at 2.3 Å resolution. The final refined structure of BEV2 is in orange and FMDV A22 in cyan.
Supplementary Figure 10 BEV2 phase refinement.
Stereo images of the electron density map around the south wall of the canyon before (a) and after (b) phase refinement by cyclic averaging. As in Supplementary Figure 9, the shown structure of BEV2 (orange) was obtained by molecular replacement using FMDV A22 as a starting model (cyan). Note the complete elimination of bias.
Supplementary Figure 11 Refined electron density map for CPV18.
Part of the electron density map (2Fo-Fc, contoured at 1σ) refined from CPV18 diffraction data, obtained by the presented method of fixed-target serial femtosecond X-ray crystallography. Image is showing residues 160 through 190.
Supplementary Figure 12 Stereo view of CPV18 electron density map.
Wall-eyed stereo image of the electron density map of the CPV18 data as shown in Supplementary Figure 11 (2Fo-Fc, contoured at 1σ).
Supplementary Figure 13 BEV2 microcrystals.
Light microscope image of a small droplet containing BEV2 microcrystals. The size of the cubic crystals is approximately 8 x 8 x 8 μm³.
Supplementary Figure 14 Indexing results for CPV18 data.
Distribution of indexed lattices for CPV18 data (data collection run 47). From the 19028 recorded images, 13424 images were regarded as a hit (more than 20 strong spots). Due to multiple hits per shot, 16739 indexing solutions were found in total. As shown above, on 4668 images more than one indexing solution was present. For 6561 images, exactly one indexing solution was found.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 and Supplementary Tables 1 and 2. (PDF 3304 kb)
Supplementary Protocol
Supplementary Protocol. (PDF 9979 kb)
Source data
Rights and permissions
About this article
Cite this article
Roedig, P., Ginn, H., Pakendorf, T. et al. High-speed fixed-target serial virus crystallography. Nat Methods 14, 805–810 (2017). https://doi.org/10.1038/nmeth.4335
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4335
This article is cited by
-
Growing and making nano- and microcrystals
Nature Protocols (2023)
-
Beef tallow injection matrix for serial crystallography
Scientific Reports (2022)
-
Polyimide mesh-based sample holder with irregular crystal mounting holes for fixed-target serial crystallography
Scientific Reports (2021)
-
Glutathione facilitates enterovirus assembly by binding at a druggable pocket
Communications Biology (2020)
-
Ultracompact 3D microfluidics for time-resolved structural biology
Nature Communications (2020)