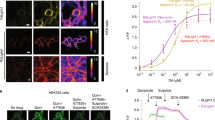
Abstract
Few tools exist to visualize and manipulate neurons that are targets of neuromodulators. We present iTango, a light- and ligand-gated gene expression system based on a light-inducible split tobacco etch virus protease. Cells expressing the iTango system exhibit increased expression of a marker gene in the presence of dopamine and blue-light exposure, both in vitro and in vivo. We demonstrated the iTango system in a behaviorally relevant context, by inducing expression of optogenetic tools in neurons under dopaminergic control during a behavior of interest. We thereby gained optogenetic control of these behaviorally relevant neurons. We applied the iTango system to decipher the roles of two classes of dopaminergic neurons in the mouse nucleus accumbens in a sensitized locomotor response to cocaine. Thus, the iTango platform allows for control of neuromodulatory circuits in a genetically and functionally defined manner with spatial and temporal precision.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Barth, A.L. Visualizing circuits and systems using transgenic reporters of neural activity. Curr. Opin. Neurobiol. 17, 567–571 (2007).
Chen, T.W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012).
Okuno, H. et al. Inverse synaptic tagging of inactive synapses via dynamic interaction of Arc/Arg3.1 with CaMKIIβ. Cell 149, 886–898 (2012).
Anderson, D.J. & Adolphs, R. A framework for studying emotions across species. Cell 157, 187–200 (2014).
Fu, Y. et al. A cortical circuit for gain control by behavioral state. Cell 156, 1139–1152 (2014).
Posner, M.I. & Petersen, S.E. The attention system of the human brain. Annu. Rev. Neurosci. 13, 25–42 (1990).
Barnea, G. et al. The genetic design of signaling cascades to record receptor activation. Proc. Natl. Acad. Sci. USA 105, 64–69 (2008).
Inagaki, H.K. et al. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell 148, 583–595 (2012).
Jagadish, S., Barnea, G., Clandinin, T.R. & Axel, R. Identifying functional connections of the inner photoreceptors in Drosophila using Tango-Trace. Neuron 83, 630–644 (2014).
Kono, M. et al. Sphingosine-1-phosphate receptor 1 reporter mice reveal receptor activation sites in vivo. J. Clin. Invest. 124, 2076–2086 (2014).
Kroeze, W.K. et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol. 22, 362–369 (2015).
Eshel, N. et al. Arithmetic and local circuitry underlying dopamine prediction errors. Nature 525, 243–246 (2015).
Herrero, J.L. et al. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature 454, 1110–1114 (2008).
Howe, M.W., Tierney, P.L., Sandberg, S.G., Phillips, P.E. & Graybiel, A.M. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature 500, 575–579 (2013).
Marlin, B.J., Mitre, M., D'amour, J.A., Chao, M.V. & Froemke, R.C. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504 (2015).
Tye, K.M. et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541 (2013).
Witten, I.B. et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330, 1677–1681 (2010).
Yagishita, S. et al. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 345, 1616–1620 (2014).
Kennedy, M.J. et al. Rapid blue-light-mediated induction of protein interactions in living cells. Nat. Methods 7, 973–975 (2010).
Wehr, M.C. et al. Monitoring regulated protein-protein interactions using split TEV. Nat. Methods 3, 985–993 (2006).
Guntas, G. et al. Engineering an improved light-induced dimer (iLID) for controlling the localization and activity of signaling proteins. Proc. Natl. Acad. Sci. USA 112, 112–117 (2015).
Williams, D.J., Puhl, H.L., III & Ikeda, S.R. Rapid modification of proteins using a rapamycin-inducible tobacco etch virus protease system. PLoS One 4, e7474 (2009).
Harper, S.M., Neil, L.C. & Gardner, K.H. Structural basis of a phototropin light switch. Science 301, 1541–1544 (2003).
Djannatian, M.S., Galinski, S., Fischer, T.M. & Rossner, M.J. Studying G protein-coupled receptor activation using split-tobacco etch virus assays. Anal. Biochem. 412, 141–152 (2011).
Gray, D.C., Mahrus, S. & Wells, J.A. Activation of specific apoptotic caspases with an engineered small-molecule-activated protease. Cell 142, 637–646 (2010).
Oades, R.D. & Halliday, G.M. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 434, 117–165 (1987).
Howe, M.W. & Dombeck, D.A. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535, 505–510 (2016).
Creed, M., Pascoli, V.J. & Lüscher, C. Addiction therapy: refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science 347, 659–664 (2015).
Lobo, M.K. & Nestler, E.J. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front. Neuroanat. 5, 41 (2011).
Pascoli, V., Turiault, M. & Lüscher, C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature 481, 71–75 (2011).
Robinson, T.E. & Berridge, K.C. Addiction. Annu. Rev. Psychol. 54, 25–53 (2003).
Lee, D. et al. Inositol 1,4,5-trisphosphate 3-kinase A is a novel microtubule-associated protein: PKA-dependent phosphoregulation of microtubule binding affinity. J. Biol. Chem. 287, 15981–15995 (2012).
Jiang, M. & Chen, G. High Ca2+-phosphate transfection efficiency in low-density neuronal cultures. Nat. Protoc. 1, 695–700 (2006).
Stoppini, L., Buchs, P.A. & Muller, D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 37, 173–182 (1991).
Woods, G. & Zito, K. Preparation of gene gun bullets and biolistic transfection of neurons in slice culture. J. Vis. Exp. (12), 675 (2008).
Oh, W.C., Hill, T.C. & Zito, K. Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proc. Natl. Acad. Sci. USA 110, E305–E312 (2013).
Gerfen, C.R., Paletzki, R. & Heintz, N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80, 1368–1383 (2013).
Acknowledgements
We thank C. Tucker (University of Colorado) for CRY2 and CIBN constructs; B. Kuhlman (University of North Carolina, Chapel Hill) for the iLID construct; B. Roth (University of North Carolina, Chapel Hill) for NPY1R, CB1R, and 5-HT1A constructs. K. Deisseroth (Stanford University) for the eNpHR-EYFP construct; R. Lefkowitz (Duke University) for the β-arrestin2 construct; S-Y. Choi (Chonnam National University) for the P2A vector; H. Zeng (Allen institute) for the TetO-EGFP construct; W. Weber (University of Freiburg) for the pSAM200 vector; and N. Gautam (Washington University in St. Louis) for the D2-YFP construct. We thank B. Lim (University of California, San Diego) for TRE-ChR2-EYFP viral production. We thank all members of the Kwon laboratory for critical discussion and support. This work was supported by funding from the Swiss National Science Foundation, the Academic Society of Geneva, the Private Foundation of the Geneva University Hospital, and a European Research Council Advanced Grant (MeSSI) (to C.L.); a Kil Chung Hee Fellowship (to D.L.); and the Max Planck Florida Institute for Neuroscience (to H.-B.K.).
Author information
Authors and Affiliations
Contributions
D.L. and H.-B.K. conceived and initiated the project. D.L. designed and made DNA constructs. D.L. performed in vitro characterization and verification. D.L., K.J., and D.J.W. performed viral injection experiments in the NAc in DAT-Cre mice. M.C. and T.S. performed viral injection and cocaine-induced locomotion sensitization experiments. M.C. performed electrophysiology recording. K.J. performed behavioral training in the ball maze and the locomotion causality test. K.J. performed in vivo time-lapse analysis, differential reward-amount testing, time-offset experiments, and image analysis in the cortical M2 area. D.L., K.J., and D.J.W. performed viral injection and histological analysis. W.C.O. performed two-photon imaging and uncaging experiments combined with electrophysiology. N.L.M. provided assistance in DNA cloning. D.L., M.C., K.J., W.C.O., C.L., and H.-B.K. wrote the manuscript. All authors discussed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The Max Planck Florida Institute for Neuroscience has filed a patent application that includes portions of the research described in this manuscript.
Integrated supplementary information
Supplementary Figure 1 Serial deletion mutants of BLITz.
(a) Design of TEVseq insertion into Jα-helix. C-terminal end of Jα-helix was serially deleted and replaced by TEVseq. TEV cleavage site is labeled as red arrowheads. Note that numbering of different BLITz is independent of deletion length. (b) Average of SEAP activity assay when transfected with different types of BLITz constructs (Dark and Blue: BLITz-2: 268.9 ± 20.5, 281.1 ± 45.2, BLITz-6: 2.21 ± 0.28, 100.7 ± 4.95, BLITz-1: 5.47 ± 0.66, 131.3 ± 5.09, BLITz-5: 1.19 ± 0.20, 5.94 ± 1.61, BLITz-4: 329.5 ± 18.4, 311.6 ± 31.6, BLITz-3: 216.1 ± 6.13, 258.4 ± 28.1, TEVseq only: 85.0 ± 7.39, 97.7 ± 3.86, No tTA: 0.29 ± 0.08, 0.34 ± 0.12). Three independent cultures were performed for each condition. DNA constructs were transfected into HEK293T cells by calcium phosphate methods. We found that the background levels of gene expression were very high when TEVseq was inserted close to the Jα-helix C-terminus (BLITz-3 and -4), indicating that TEVseq was accessible to TEV protease. Removing two more amino acids from Jα-helix (BLITz-1 and -6), completely abolished baseline gene expression, while maintaining high light-induced gene expression. Interestingly, removing amino acids 137 and 138 (BLITz-2) restored the baseline gene expression, suggesting those amino acids are tied to the light-dependent conformational changes. We subsequently tested intact TEVseq without fusion to AsLOV2 protein (similar to classical Tango system) and observed that basal gene expression was high, as expected, and subsequent fold change was minimal (~1.4 fold). Removal of TetR-VP16 (No tTA) led to nearly undetectable signals, suggesting that background gene expression did not originate from the reporter itself. (c) Fold induction changes when blue light was illuminated.
Supplementary Figure 2 Optimization of BLITz in various conditions of transfection ratios.
(a) Schematic experimental procedures in HEK293T cells. Right before blue light activation, media was replaced by fresh DMEM containing 10% FBS. (b) Summary graph of SEAP assay at different ratios of BLITz constructs. Notably, 8:8:2 (TM-CIBN-TEV-N-AsLOV2-tTA: CRY2-TEV-C: TetO-SEAP) ratio shows the best fold changes. Error bar represents ± S.D. of three independent experiments.
Supplementary Figure 3 Blue-light-dependent EGFP expression of BLITz in HEK293T.
(a) Schematic designs of DNA constructs. TdTomato was separately transfected for a transfection marker. (b) Representative images of gene expression by BLITz. Parts of images are same as Fig. 1b. Images at Blue light 5 min and 20 min are additionally supplemented. Dashed boxes were magnified (4x) for clarification. Expression pattern of EGFP are very similar to the pattern of SEAP assay (Fig. 1c). Scale bar, 200 μm (1x) and 50 μm (4x magnified images).
Supplementary Figure 4 Two-color flow cytometry analysis of EGFP reporter expression in the BLITz system.
(a-h) Scatter plots of two-color (y-axis: TdTomato, x-axis: EGFP) flow cytometry analysis in HEK293T transfected with BLITz systems. Analyzed cells were divided into four quadrants (Black, Magenta, Turquoise, and Yellow) by EGFP and TdTomato fluorescent intensity. Distribution of four quadrants are displayed as bar graph. The right upper quadrant suggests both high TdTomato and EGFP expression. Confocal images are same as Fig. 1b. Scale bar, 200 μm.
Supplementary Figure 5 Optimization of DRD2-iTango at various transfection ratios.
(a) Schematics of DNA constructs of DRD2-iTango. (b) SEAP assay shows different gene expression level of DRD2-iTango at various transfection ratios. To test DRD2-iTango, we treated 10 μM Quinpirole and shined 10-second pulsed blue light for 12 hrs. Note that whenever high ratio of β-Arrestin2-TEV-N was transfected, fold change of gene expression was higher. The summary graph is represented by means ± S.D. of three independent measurements.
Supplementary Figure 6 Scatter-plot analysis of blue-light- and agonist-dependent EGFP expression in DRD2-iTango2.
(a) Schematics of DNA constructs of DRD2-iTango2. (b-i) Two-color scatter plots (x-axis: TdTomato, y-axis: EGFP) of hippocampal culture neuron expressing DRD2-iTango2. Analyzed cells were divided by four quadrants (Black, Magenta, Turquoise, and Yellow) and their distributional percentage was quantified by horizontal histograms. Red dot cross lines indicate threshold for defining four quadrants.
Supplementary Figure 7 Agonist dose-dependent expression of EGFP reporter in DRD2-iTango2.
(a-f) Representative confocal images (left panel), scatter plots of two-color (middle panel; y-axis: TdTomato, x-axis: EGFP), and distribution graph (right panel) of DRD2-iTango2 with different concentration of quinpirole and haloperidol in blue light condition. Analyzed cells were divided by four quadrants (Black, Magenta, Turquoise, and Yellow) and their distributional percentage was displayed as vertical histograms. Red dot cross lines indicate threshold for defining four quadrants.Scale bar, 100 μm. (g) Summary graph of average G/R (Control: 0.15 ± 0.03, Quinpirole 10 nM: 0.55 ± 0.02, Quinpirole 100 nM: 0.67 ± 0.03, Quinpirole 1 μM: 1.00 ± 0.09, Quinpirole 10 μM: 1.28 ± 0.04, and Quinpirole 10 μM + Haloperidol 2.5 μM: 0.11 ± 0.03; 3 independent experiments, Mean ± S.E.M; oneway-ANOVA p<0.001; Bonferroni post-hoc test; ***p < 0.001) and fold changes.
Supplementary Figure 8 Complete elimination of DRD2-iTango2 activity by further deletion of C-terminal amino acids of TEV-C.
(a, b) Schematic protein structure of full-length TEV protease and C-terminal amino acid sequences of deleted mutants of TEV-C (TEV-C 86, TEV-C 91, TEV-C 95; numbers indicate the position of last amino acid within the deleted mutant). To optimize the minimum sequence of TEV-C for DRD2-iTango2 system without losing intact protease activity, we generated three mutants of β-Arrestin2-TEV-C-P2A-TdTomato by deleting C-terminal amino acids of TEV-C. N-terminal intact region are colored with red and deleted region are colored with light gray, dark gray, and black. (c) Deleted iTango2 mutant constructs or TEV-N as control were transfected into HEK293T cells. After blue light (2 hrs) and 10 μM quinpirole treatment, SEAP activity was measured to check the level of gene expression induced by TEV-protease-dependent cleavage. TEV protease function is completely abolished in three C-terminal deletion mutants (TEV-C 86: 3.08 ± 1.01, TEV-C 91: 3.96 ± 0.81, TEV-C 95: 6.45 ± 1.00) or TEV-N (0.88 ± 0.06), but remains in the original truncated version of TEV-C (340.87 ± 33.53). Mean ± S.D.
Supplementary Figure 9 No changes in excitatory-synapse numbers and functions by iTango2.
(a) Schematic drawing of experiments. Layer 2/3 pyramidal neurons with DRD2-iTango2 transfected or neighboring control neurons were compared. (b) iTango2 transfected neuron was identified by TdTomato signal originated from β-Arrestin2-TEV-C-P2A-TdTomato. In addition, gold particle was observed from DNA transfected neurons. (c) Representative images of dendritic spines from control and iTango2 transfected neurons. To visualize spine morphology clearly, Alexa-488 (200 μM) was added into the recording pipette. (d) Average graph of spine density (Control: 0.93 ± 0.07, 5 neurons, 2.8 ROI / neuron; iTango2: 0.94 ± 0.06, 8 neurons, 2.6 ROI / neuron, p > 0.1). (e) Target neuron image and dendritic spine for glutamate uncaging. Cross indicates glutamate uncaging spot. Representative traces of uncaging induced AMPAR-uEPSCs. Arrow indicates uncaging time. 2.5 mM MNI-Glutamate was perfused in normal ACSF. Spontaneous activity was blocked by 1 μM TTX. 1 ms pulse of 720 nm laser was used for glutamate photolysis. (f) Relationship between uEPSCs and spine size (Control with small size: 160.87 ± 9.23; iTango with small size: 136.94 ± 20.20, p > 0.05; Control with large size: 477.78 ± 85.1; iTango with large size: 525.50 ± 66.89, p > 0.05). (g) The amplitude of uEPSCs grouped by spine size was compared between control and iTango2 transfected neurons (Control: 3 neurons, 20 spines; iTango2: 6 neurons, 24 spines; Control with small size: 5.50 ± 0.39; iTango2 with small size: 5.31 ± 0.94, p > 0.1; Control with large size: 12.34 ± 0.84; iTango with large size: 12.44 ± 0.78, p > 0.1).
Supplementary Figure 10 Scatter-plot analysis of NPY1R-iTango2, CB1R-iTango2, and 5-HT1A-iTango2.
(a1-4) Scatter plots (x-axis: TdTomato, y-axis: EGFP) of hippocampal culture neuron expressing NPY1R-iTango2. (b1-4) Scatter plots of CB1R-iTango2. (c1-4) Scatter plots of 5-HT1A-iTango2. Analyzed cells were divided by four quadrants (Black, Magenta, Turquoise, and Yellow) and their distributional percentage was quantified by horizontal histograms. The right upper quadrant (Yellow) represents both high TdTomato and EGFP expression. Red dot cross lines indicate threshold for defining four quadrants.
Supplementary Figure 11 Time course of iTango2 reporter-gene expression in vitro and in vivo.
(a) Experimental time line of DRD2-iTango2 in hippocampal culture neurons. (b) Percentage change of Yellow categorized neurons over time (upper right quadrant). (c) Two-color scatter plots (x-axis: TdTomato, y-axis: EGFP) of DRD2-iTango2. Analyzed cells were divided by four categories (Turquoise, Yellow, Black, and Magenta). Division of categories is indicated as red dot cross lines. Total cell numbers of each group are as following: (0 hour: 138 neurons, 8 hour: 137 neurons, 24 hour: 173 neurons, 72 hour: 133 neurons, 120 hour: 152 neurons, 240 hour: 208 neurons). (d) Schematic figure of virus injection. (e) Summary graph of G/R ratio changes after induction of DRD2-iTango labeling (24 hour: 0.238 ± 0.065, 6 mice; 48 hour: 0.489 ± 0.079, 6 mice; 96 hour: 0.295 ± 0.064, 6 mice; 192 hour: 0.244 ± 0.049, 6 mice; Fhour = 3.32, p < 0.05). (f) Coronal sections of the NAc respectively from left to right imaged 24, 48, 96, and 192 hour after induction of iTango2 labeling. Scale bar, 100 μm.
Supplementary Figure 12 Time offset analysis of DRD2-iTango2 labeling.
(a-e) Schematics represent experimental design of in vitro time offset experiment. Representative images (left panel) and scatter plots (right panel, y axis: EGFP, x axis: TdTomato) of EGFP expression in time offset experiment of iTango2. (f) Analyzed cells were divided by four quadrants (Black, Magenta, Turquoise, and Yellow) and their distributional percentage was displayed as horizontal histograms. (g) Schematic figures of virus injection and experiment setup. (h) Schematic of experiment; (1) blue light in sync with reward, (2) blue light out of sync with reward, (3) reward alone, or (4) blue light alone was administered to induce the expression of EGFP reporter. (i) Summary graph of G/R ratio in NAc (Synchronous delivery: 0.446 ± 0.104, 6 mice; Asynchronous delivery: 0.135 ± 0.084, 6 mice; Reward control: 0.034 ± 0.007, 6 mice; Blue light control: 0.035 ± 0.019, 6 mice; *p < 0.05, **p < 0.01). (j) Coronal sections of the NAc from mice treated with synchronous delivery, asynchronous delivery, reward alone, or blue light alone, respectively from top to bottom. Scale bar, 100 μm.
Supplementary Figure 13 In vivo reward-amount-dependent iTango2 labeling in the NAc.
(a) Schematic figures of virus injection and experiment setup. (b) Schematic of experiment; blue light was administered in sync with different amounts of reward (large reward: 16 μl, small reward: 4 μl, or no reward) to induce the expression of EGFP reporter. (c) Summary graph of G/R ratio in NAc (Large reward: 0.662 ± 0.070, 5 mice; Small reward: 0.382 ± 0.033, 6 mice; No reward: 0.114 ± 0.070, 6 mice; **p < 0.01, ***p < 0.001). (d) Quantification of infected neurons expressing tdTomato alone, EGFP alone, or co-expressing both fluorescent proteins. (e) Coronal sections of the NAc from mice receiving large reward (top), small reward (middle), or no reward (bottom). Scale bar, 100 μm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14. (PDF 3947 kb)
Supplementary Data
DNA sequences. (PDF 880 kb)
Rights and permissions
About this article
Cite this article
Lee, D., Creed, M., Jung, K. et al. Temporally precise labeling and control of neuromodulatory circuits in the mammalian brain. Nat Methods 14, 495–503 (2017). https://doi.org/10.1038/nmeth.4234
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4234
This article is cited by
-
A programmable protease-based protein secretion platform for therapeutic applications
Nature Chemical Biology (2024)
-
A single-component, light-assisted uncaging switch for endoproteolytic release
Nature Chemical Biology (2024)
-
Parkinson’s disease tremor prediction using EEG data analysis-A preliminary and feasibility study
BMC Neurology (2023)
-
The neural circuit that makes maternal mice respond to pups’ cries
Nature (2023)
-
In vivo recording of the circadian calcium rhythm in Prokineticin 2 neurons of the suprachiasmatic nucleus
Scientific Reports (2023)