Abstract
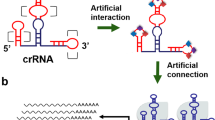
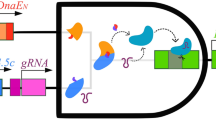
The complex phenotypes of eukaryotic cells are controlled by decision-making circuits and signaling pathways. A key obstacle to implementing artificial connections in signaling networks has been the lack of synthetic devices for efficient sensing, processing and control of biological signals. By extending sgRNAs to include modified riboswitches that recognize specific signals, we can create CRISPR–Cas9-based 'signal conductors' that regulate transcription of endogenous genes in response to external or internal signals of interest. These devices can be used to construct all the basic types of Boolean logic gates that perform logical signal operations in mammalian cells without needing the layering of multiple genetic circuits. They can also be used to rewire cellular signaling events by constructing synthetic links that couple different signaling pathways. Moreover, this approach can be applied to redirect oncogenic signal transduction by controlling simultaneous bidirectional (ON–OFF) gene transcriptions, thus enabling reprogramming of the fate of cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Scott, J.D. & Pawson, T. Cell signaling in space and time: where proteins come together and when they're apart. Science 326, 1220–1224 (2009).
Riento, K. & Ridley, A.J. Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 4, 446–456 (2003).
Bhattacharyya, R.P., Reményi, A., Yeh, B.J. & Lim, W.A. Domains, motifs, and scaffolds: the role of modular interactions in the evolution and wiring of cell signaling circuits. Annu. Rev. Biochem. 75, 655–680 (2006).
Slusarczyk, A.L., Lin, A. & Weiss, R. Foundations for the design and implementation of synthetic genetic circuits. Nat. Rev. Genet. 13, 406–420 (2012).
Youk, H. & Lim, W.A. Secreting and sensing the same molecule allows cells to achieve versatile social behaviors. Science 343, 1242782 (2014).
Townshend, B., Kennedy, A.B., Xiang, J.S. & Smolke, C.D. High-throughput cellular RNA device engineering. Nat. Methods 12, 989–994 (2015).
Chau, A.H., Walter, J.M., Gerardin, J., Tang, C. & Lim, W.A. Designing synthetic regulatory networks capable of self-organizing cell polarization. Cell 151, 320–332 (2012).
Galloway, K.E., Franco, E. & Smolke, C.D. Dynamically reshaping signaling networks to program cell fate via genetic controllers. Science 341, 1235005 (2013).
Hsu, P.D., Lander, E.S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Qi, L.S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013).
Maeder, M.L. et al. CRISPR RNA-guided activation of endogenous human genes. Nat. Methods 10, 977–979 (2013).
Mali, P. et al. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 31, 833–838 (2013).
Zalatan, J.G. et al. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell 160, 339–350 (2015).
Polstein, L.R. & Gersbach, C.A. A light-inducible CRISPR-Cas9 system for control of endogenous gene activation. Nat. Chem. Biol. 11, 198–200 (2015).
Nihongaki, Y., Yamamoto, S., Kawano, F., Suzuki, H. & Sato, M. CRISPR-Cas9-based photoactivatable transcription system. Chem. Biol. 22, 169–174 (2015).
Boerneke, M.A. & Hermann, T. Ligand-responsive RNA mechanical switches. RNA Biol. 12, 780–786 (2015).
Bayer, T.S. & Smolke, C.D. Programmable ligand-controlled riboregulators of eukaryotic gene expression. Nat. Biotechnol. 23, 337–343 (2005).
Culler, S.J., Hoff, K.G. & Smolke, C.D. Reprogramming cellular behavior with RNA controllers responsive to endogenous proteins. Science 330, 1251–1255 (2010).
Moon, T.S., Lou, C., Tamsir, A., Stanton, B.C. & Voigt, C.A. Genetic programs constructed from layered logic gates in single cells. Nature 491, 249–253 (2012).
Green, C. Restore Wild-Type Functions to P53 Mutants Using an RNA-Based Combinatorial Approach. Report No. DAMD17-97-1-7036 (SRI International, 2000).
Zhao, X. et al. An RNA aptamer that interferes with the DNA binding of the HSF transcription activator. Nucleic Acids Res. 34, 3755–3761 (2006).
Jian, Y. et al. RNA aptamers interfering with nucleophosmin oligomerization induce apoptosis of cancer cells. Oncogene 28, 4201–4211 (2009).
Tsui, K.H., Cheng, A.J., Chang, Pe., Pan, T.L. & Yung, B.Y. Association of nucleophosmin/B23 mRNA expression with clinical outcome in patients with bladder carcinoma. Urology 64, 839–844 (2004).
Kaur, J. & Tikoo, K. Ets1 identified as a novel molecular target of RNA aptamer selected against metastatic cells for targeted delivery of nano-formulation. Oncogene 34, 5216–5228 (2015).
Xu, W. & Ellington, A.D. Anti-peptide aptamers recognize amino acid sequence and bind a protein epitope. Proc. Natl. Acad. Sci. USA 93, 7475–7480 (1996).
Lee, Y.J. & Lee, S.W. Regression of hepatocarcinoma cells using RNA aptamer specific to alpha-fetoprotein. Biochem. Biophys. Res. Commun. 417, 521–527 (2012).
Luo, X., Budihardjo, I., Zou, H., Slaughter, C. & Wang, X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94, 481–490 (1998).
Beisel, C.L., Bayer, T.S., Hoff, K.G. & Smolke, C.D. Model-guided design of ligand-regulated RNAi for programmable control of gene expression. Mol. Syst. Biol. 4, 224 (2008).
An, C.I., Trinh, V.B. & Yokobayashi, Y. Artificial control of gene expression in mammalian cells by modulating RNA interference through aptamer-small molecule interaction. RNA 12, 710–716 (2006).
Wittmann, A. & Suess, B. Engineered riboswitches: expanding researchers' toolbox with synthetic RNA regulators. FEBS Lett. 586, 2076–2083 (2012).
Winkler, W., Nahvi, A. & Breaker, R.R. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 419, 952–956 (2002).
Yen, L. et al. Exogenous control of mammalian gene expression through modulation of RNA self-cleavage. Nature 431, 471–476 (2004).
Chen, Y.Y., Jensen, M.C. & Smolke, C.D. Genetic control of mammalian T-cell proliferation with synthetic RNA regulatory systems. Proc. Natl. Acad. Sci. USA 107, 8531–8536 (2010).
Shechner, D.M., Hacisuleyman, E., Younger, S.T. & Rinn, J.L. Multiplexable, locus-specific targeting of long RNAs with CRISPR-Display. Nat. Methods 12, 664–670 (2015).
Nishimasu, H. et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156, 935–949 (2014).
Fu, Y., Sander, J.D., Reyon, D., Cascio, V.M. & Joung, J.K. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 32, 279–284 (2014).
Doench, J.G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Slaymaker, I.M. et al. Rationally engineered Cas9 nucleases with improved specificity. Science 351, 84–88 (2016).
Thakore, P.I. et al. Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat. Methods 12, 1143–1149 (2015).
Hilton, I.B. et al. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 33, 510–517 (2015).
Gilbert, L.A. et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159, 647–661 (2014).
Acknowledgements
We are indebted to the donors whose names were not included in the author list, but who participated in this program. This work was supported by the National Key Basic Research Program of China (973 Program) (2014CB745201 to Z.C.), National Natural Science Foundation of China (81402103 to Y.L.), the Shenzhen Municipal Government of China (ZDSYS201504301722174, JCYJ20150330102720130 and GJHZ20150316154912494 to W.H.), Special Support Funds of Shenzhen for Introduced High-Level Medical Team to W.H., and Shenzhen High Level Medical Discipline Development Program (2016031638 to W.H. and Z.C.).
Author information
Authors and Affiliations
Contributions
Y.L., Y.Z., Z.C., A.H., J.L., H.W., L.L., C.Z., J.L., X.G. and Q.Z. performed experiments and data analysis. Y.L., W.H. and Z.C. designed and supervised the project and wrote the paper. W.H. and Z.C. provided financial support for the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–36 and Supplementary Tables 1–5. (PDF 5395 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Zhan, Y., Chen, Z. et al. Directing cellular information flow via CRISPR signal conductors. Nat Methods 13, 938–944 (2016). https://doi.org/10.1038/nmeth.3994
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3994
This article is cited by
-
Optogenetic control of RNA function and metabolism using engineered light-switchable RNA-binding proteins
Nature Biotechnology (2022)
-
CRISPR signal conductor 2.0 for redirecting cellular information flow
Cell Discovery (2022)
-
Engineering synthetic RNA devices for cell control
Nature Reviews Genetics (2022)
-
Reprogrammed tracrRNAs enable repurposing of RNAs as crRNAs and sequence-specific RNA biosensors
Nature Communications (2022)
-
Endogenous promoter-driven sgRNA for monitoring the expression of low-abundance transcripts and lncRNAs
Nature Cell Biology (2021)