Abstract
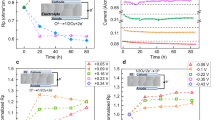
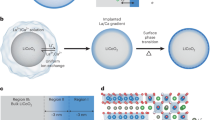
Improvement of solid oxide fuel cells strongly relies on the development of cathode materials with high catalytic activity for the oxygen reduction reaction. Excellent activity was found for perovskite-type oxides such as La1−xSrxCoO3−δ (LSC), but performance degradation, probably caused by surface composition changes, hinders exploitation of the full potential of LSC. This study reveals that the potentially very high activity of the LSC surface can be traced back to few very active sites. Already tiny amounts of SrO, for example, 4% of a monolayer, deposited on an LSC surface, lead to severe deactivation. Co, on the other hand, causes (re-)activation, suggesting that active sites are strongly related to Co being present at the surface. These insights could be gained by a novel method to measure changes of the electrochemical performance of thin film electrodes in situ, while modifying their surface: impedance spectroscopy measurements during deposition of well-defined fractions of monolayers of Sr-, Co- and La-oxides by single laser pulses in a pulsed laser deposition chamber.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Boukamp, B. A. Fuel cells: the amazing perovskite anode. Nat. Mater. 2, 294–296 (2003).
Choi, S. et al. Highly efficient and robust cathode materials for low-temperature solid oxide fuel cells: PrBa0.5Sr0.5Co2−xFexO5+δ . Sci. Rep. 3, 2426 (2013).
Shao, Z. & Haile, S. M. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431, 170–173 (2004).
Rupp, G. M., Schmid, A., Nenning, A. & Fleig, J. The superior properties of La0.6Ba0.4CoO3−δ thin film electrodes for oxygen exchange in comparison to La0.6Sr0.4CoO3−δ . J. Electrochem. Soc. 163, F564–F573 (2016).
Baumann, F. S. et al. Quantitative comparison of mixed conducting SOFC cathode materials by means of thin film model electrodes. J. Electrochem. Soc. 154, B931–B941 (2007).
Cai, Z., Kubicek, M., Fleig, J. & Yildiz, B. Chemical heterogeneities on La0.6Sr0.4CoO3−δ thin films—correlations to cathode surface activity and stability. Chem. Mater. 24, 1116–1127 (2012).
De Souza, R. A. & Kilner, J. A. Oxygen transport in La1−xSrxMn1−yCoyO3+/−δ perovskites—Part I. Oxygen tracer diffusion. Solid State Ion. 106, 175–187 (1998).
Van der Haar, L. M., Den Otter, M. W., Morskate, M., Bouwmeester, H. J. M. & Verweij, H. Chemical diffusion and oxygen surface transfer of La1−xSrxCoO3−δ studied with electrical conductivity relaxation. J. Electrochem. Soc. 149, J41–J46 (2002).
La O, G. J. et al. Catalytic activity enhancement for oxygen reduction on epitaxial perovskite thin films for solid-oxide fuel cells. Angew. Chem. Int. Ed. 49, 5344–5347 (2010).
Rupp, G. M. et al. Surface chemistry of La0.6Sr0.4CoO3−δ thin films and its impact on the oxygen surface exchange resistance. J. Mater. Chem. A 3, 22759–22769 (2015).
Huber, A.-K. et al. In situ study of activation and de-activation of LSM fuel cell cathodes—electrochemistry and surface analysis of thin-film electrodes. J. Catalys. 294, 79–88 (2012).
Wang, W. & Jiang, S. P. A mechanistic study on the activation process of (La, Sr)MnO3 electrodes of solid oxide fuel cells. Solid State Ion. 177, 1361–1369 (2006).
Hu, B., Mahapatra, M. K. & Singh, P. Performance regeneration in lanthanum strontium manganite cathode during exposure to H2O and CO2 containing ambient air atmospheres. J. Ceram. Soc. Japan 123, 199–204 (2015).
Kubicek, M., Limbeck, A., Fromling, T., Hutter, H. & Fleig, J. Relationship between cation segregation and the electrochemical oxygen reduction kinetics of La0.6Sr0.4CoO3−δ thin film electrodes. J. Electrochem. Soc. 158, B727–B734 (2011).
Baumann, F. S. et al. Strong performance improvement of La0.6Sr0.4Co0.8Fe0.2O3−δ SOFC cathodes by electrochemical activation. J. Electrochem. Soc. 152, A2074–A2079 (2005).
Wang, H. et al. Mechanisms of performance degradation of (La, Sr)(Co, Fe)O3−δ solid oxide fuel cell cathodes. J. Electrochem. Soc. 163, F581–F585 (2016).
Oh, D., Gostovic, D. & Wachsman, E. D. Mechanism of La0.6Sr0.4Co0.2Fe0.8O3 cathode degradation. J. Mater. Res. 27, 1992–1999 (2012).
Chen, Y. et al. Impact of Sr segregation on the electronic structure and oxygen reduction activity of SrTi1−xFexO3 surfaces. Energy Environ. Sci. 5, 7979–7988 (2012).
Jung, W. & Tuller, H. L. Investigation of surface Sr segregation in model thin film solid oxide fuel cell perovskite electrodes. Energy Environ. Sci. 5, 5370–5378 (2012).
Kim, Y.-M., Chen, X., Jiang, S. P. & Bae, J. Effect of strontium content on chromium deposition and poisoning in Ba1−xSrxCo0.8Fe0.2O3−δ (0.3 ≤ x ≥ 0.7) cathodes of solid oxide fuel cells. J. Electrochem. Soc. 159, B185–B194 (2011).
Bucher, E., Gspan, C., Hofer, F. & Sitte, W. Sulphur poisoning of the SOFC cathode material La0.6Sr0.4CoO3−δ . Solid State Ion. 238, 15–23 (2013).
Bucher, E., Sitte, W., Klauser, F. & Bertel, E. Impact of humid atmospheres on oxygen exchange properties, surface-near elemental composition, and surface morphology of La0.6Sr0.4CoO3−δ . Solid State Ion. 208, 43–51 (2012).
Crumlin, E. J. et al. Oxygen electrocatalysis on epitaxial La0.6Sr0.4CoO3−δ Perovskite thin films for solid oxide fuel cells. J. Electrochem. Soc. 159, F219–F225 (2012).
Wilson, J. R., Sase, M., Kawada, T. & Adler, S. B. Measurement of oxygen exchange kinetics on thin-film La0.6Sr0.4CoO3−δ using nonlinear electrochemical impedance spectroscopy. Electrochem. Solid-State Lett. 10, B81–B86 (2007).
Baumann, F. S., Maier, J. & Fleig, J. The polarization resistance of mixed conducting SOFC cathodes: a comparative study using thin film model electrodes. Solid State Ion. 179, 1198–1204 (2008).
Kawada, T. et al. Determination of oxygen vacancy concentration in a thin film of La0.6Sr0.4CoO3−δ by an electrochemical method. J. Electrochem. Soc. 149, E252–E259 (2002).
Tsvetkov, N., Lu, Q., Sun, L., Crumlin, E. J. & Yildiz, B. Improved chemical and electrochemical stability of perovskite oxides with less reducible cations at the surface. Nat. Mater. 15, 1010–1016 (2016).
Yu, A. S., Küngas, R., Vohs, J. M. & Gorte, R. J. Modification of SOFC cathodes by atomic layer deposition. J. Electrochem. Soc. 160, F1225–F1231 (2013).
Crumlin, E. J. et al. Surface strontium enrichment on highly active perovskites for oxygen electrocatalysis in solid oxide fuel cells. Energy Environ. Sci. 5, 6081–6088 (2012).
Mutoro, E., Crumlin, E. J., Biegalski, M. D., Christen, H. M. & Shao-Horn, Y. Enhanced oxygen reduction activity on surface-decorated perovskite thin films for solid oxide fuel cells. Energy Environ. Sci. 4, 3689–3696 (2011).
Kuhn, M., Hashimoto, S., Sato, K., Yashiro, K. & Mizusaki, J. Oxygen nonstoichiometry and thermo-chemical stability of La0.6Sr0.4CoO3−δ . J. Solid State Chem. 197, 38–45 (2013).
Kubicek, M. et al. Cation diffusion in La0.6Sr0.4CoO3−δ below 800 °C and its relevance for Sr segregation. Phys. Chem. Chem. Phys. 16, 2715–2726 (2014).
Baumann, F. S., Fleig, J., Habermeier, H. & Maier, J. Impedance spectroscopic study on well-defined (La, Sr)(Co, Fe)O3−δ model electrodes. Solid State Ion. 177, 1071–1081 (2006).
Hjalmarsson, P., Søgaard, M. & Mogensen, M. Electrochemical performance and degradation of (La0.6Sr0.4)0.99CoO3−δ as porous SOFC-cathode. Solid State Ion. 179, 1422–1426 (2008).
Chen, Y. et al. Segregated chemistry and structure on (001) and (100) surfaces of (La1−xSrx)2CoO4 override the crystal anisotropy in oxygen exchange kinetics. Chem. Mater. 27, 5436–5450 (2015).
Mogensen, M. B. et al. New hypothesis for SOFC ceramic oxygen electrode mechanisms. ECS Trans. 72, 93–103 (2016).
Maier, J. in Physical Chemistry of Ionic Materials: Ions and Electrons in Solids Ch. 7.3, 433–434 (John Wiley, 2004).
Pechini, M. P. Method of preparing lead and alkaline earth titanates and niobates and coating method using the same to form a capacitor. US patent 3330697 A (1967).
Rupp, G. M. et al. Correlating surface cation composition and thin film microstructure with the electrochemical performance of lanthanum strontium cobaltite (LSC) electrodes. J. Mater. Chem. A 2, 7099–7108 (2014).
Limbeck, A. et al. Dynamic etching of soluble surface layers with on-line inductively coupled plasma mass spectrometry detection—a novel approach for determination of complex metal oxide surface cation stoichiometry. J. Anal. Atom. Spectr. 31, 1638–1646 (2016).
Kubicek, M. et al. Tensile lattice strain accelerates oxygen surface exchange and diffusion in La1−xSrxCoO3−δ thin films. ACS Nano 7, 3276–3286 (2013).
Acknowledgements
The authors gratefully acknowledge funding by Austrian Science Fund (FWF) project P4509-N16 and W1243-N16. Scanning electron microscope images were obtained using facilities at the University Service Centre for Transmission Electron Microscopy, Vienna University of Technology, Austria.
Author information
Authors and Affiliations
Contributions
G.M.R. prepared the samples, developed the IPLD setup, performed and analysed impedance, X-ray diffraction and ICP measurements and wrote the manuscript. A.K.O. had the initial idea for the IPLD setup, supported the development and was involved at all stages of the study from planning to data analysis and discussion. A.N. performed XPS measurements and analysis. A.L. supervised the ICP measurements and was involved in the interpretation of the data. J.F. supervised all impedance spectroscopy measurements and was involved in the interpretation of the results as well as the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1615 kb)
Rights and permissions
About this article
Cite this article
Rupp, G., Opitz, A., Nenning, A. et al. Real-time impedance monitoring of oxygen reduction during surface modification of thin film cathodes. Nature Mater 16, 640–645 (2017). https://doi.org/10.1038/nmat4879
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4879
This article is cited by
-
Engineering surface dipoles on mixed conducting oxides with ultra-thin oxide decoration layers
Nature Communications (2024)
-
Perovskite Cathode Materials for Low-Temperature Solid Oxide Fuel Cells: Fundamentals to Optimization
Electrochemical Energy Reviews (2022)
-
Acidity of surface-infiltrated binary oxides as a sensitive descriptor of oxygen exchange kinetics in mixed conducting oxides
Nature Catalysis (2020)
-
Surface Segregation in Solid Oxide Cell Oxygen Electrodes: Phenomena, Mitigation Strategies and Electrochemical Properties
Electrochemical Energy Reviews (2020)
-
Factors controlling surface oxygen exchange in oxides
Nature Communications (2019)