Abstract
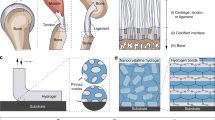
In many animals, the bonding of tendon and cartilage to bone is extremely tough (for example, interfacial toughness ∼800 J m−2; refs 1,2), yet such tough interfaces have not been achieved between synthetic hydrogels and non-porous surfaces of engineered solids3,4,5,6,7,8,9. Here, we report a strategy to design tough transparent and conductive bonding of synthetic hydrogels containing 90% water to non-porous surfaces of diverse solids, including glass, silicon, ceramics, titanium and aluminium. The design strategy is to anchor the long-chain polymer networks of tough hydrogels covalently to non-porous solid surfaces, which can be achieved by the silanation of such surfaces. Compared with physical interactions, the chemical anchorage results in a higher intrinsic work of adhesion and in significant energy dissipation of bulk hydrogel during detachment, which lead to interfacial toughness values over 1,000 J m−2. We also demonstrate applications of robust hydrogel–solid hybrids, including hydrogel superglues, mechanically protective hydrogel coatings, hydrogel joints for robotic structures and robust hydrogel–metal conductors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bobyn, J., Wilson, G., MacGregor, D., Pilliar, R. & Weatherly, G. Effect of pore size on the peel strength of attachment of fibrous tissue to porous-surfaced implants. J. Biomed. Mater. Res. 16, 571–584 (1982).
Moretti, M. et al. Structural characterization and reliable biomechanical assessment of integrative cartilage repair. J. Biomech. 38, 1846–1854 (2005).
Gong, J. P., Katsuyama, Y., Kurokawa, T. & Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 15, 1155–1158 (2003).
Wang, Q. et al. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 463, 339–343 (2010).
Henderson, K. J., Zhou, T. C., Otim, K. J. & Shull, K. R. Ionically cross-linked triblock copolymer hydrogels with high strength. Macromolecules 43, 6193–6201 (2010).
Sun, J.-Y. et al. Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012).
Sun, T. L. et al. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nature Mater. 12, 932–937 (2013).
Kamata, H., Akagi, Y., Kayasuga-Kariya, Y., Chung, U.-i. & Sakai, T. “Nonswellable” hydrogel without mechanical hysteresis. Science 343, 873–875 (2014).
Liu, M. et al. An anisotropic hydrogel with electrostatic repulsion between cofacially aligned nanosheets. Nature 517, 68–72 (2015).
Peppas, N. A., Hilt, J. Z., Khademhosseini, A. & Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 18, 1345–1360 (2006).
Lee, K. Y. & Mooney, D. J. Hydrogels for tissue engineering. Chem. Rev. 101, 1869–1880 (2001).
Sidorenko, A., Krupenkin, T., Taylor, A., Fratzl, P. & Aizenberg, J. Reversible switching of hydrogel-actuated nanostructures into complex micropatterns. Science 315, 487–490 (2007).
Banerjee, I., Pangule, R. C. & Kane, R. S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 23, 690–718 (2011).
Dong, L., Agarwal, A. K., Beebe, D. J. & Jiang, H. Adaptive liquid microlenses activated by stimuli-responsive hydrogels. Nature 442, 551–554 (2006).
Beebe, D. J. et al. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature 404, 588–590 (2000).
Keplinger, C. et al. Stretchable, transparent, ionic conductors. Science 341, 984–987 (2013).
Yu, C. et al. Electronically programmable, reversible shape change in two-and three-dimensional hydrogel structures. Adv. Mater. 25, 1541–1546 (2013).
Kurokawa, T., Furukawa, H., Wang, W., Tanaka, Y. & Gong, J. P. Formation of a strong hydrogel–porous solid interface via the double-network principle. Acta Biomater. 6, 1353–1359 (2010).
Ahagon, A. & Gent, A. Effect of interfacial bonding on the strength of adhesion. J. Polym. Sci. 13, 1285–1300 (1975).
Gent, A. Adhesion and strength of viscoelastic solids. Is there a relationship between adhesion and bulk properties? Langmuir 12, 4492–4496 (1996).
Kaelble, D. Peel adhesion: Influence of surface energies and adhesive rheology. J. Adhes. 1, 102–123 (1969).
Derail, C., Allal, A., Marin, G. & Tordjeman, P. Relationship between viscoelastic and peeling properties of model adhesives. Part 1. Cohesive fracture. J. Adhes. 61, 123–157 (1997).
Sudre, G., Olanier, L., Tran, Y., Hourdet, D. & Creton, C. Reversible adhesion between a hydrogel and a polymer brush. Soft Matter 8, 8184–8193 (2012).
Weissman, J. M., Sunkara, H. B., Albert, S. T. & Asher, S. A. Thermally switchable periodicities and diffraction from mesoscopically ordered materials. Science 274, 959–963 (1996).
Gong, J. P. Why are double network hydrogels so tough? Soft Matter 6, 2583–2590 (2010).
Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 10, 672–687 (2014).
Lake, G. J. & Thomas, A. G. Strength of highly elastic materials. Proc. R. Soc. Lond. Ser. A 300, 108–119 (1967).
Webber, R. E., Creton, C., Brown, H. R. & Gong, J. P. Large strain hysteresis and Mullins effect of tough double-network hydrogels. Macromolecules 40, 2919–2927 (2007).
Tegelström, H. & Wyöni, P. I. Silanization of supporting glass plates avoiding fixation of polyacrylamide gels to glass cover plates. Electrophoresis 7, 99 (1986).
Kendall, K. Thin-film peeling-the elastic term. J. Phys. D 8, 1449–1452 (1975).
Ghatak, A., Chaudhury, M. K., Shenoy, V. & Sharma, A. Meniscus instability in a thin elastic film. Phys. Rev. Lett. 85, 4329–4332 (2000).
Biggins, J. S., Saintyves, B., Wei, Z., Bouchaud, E. & Mahadevan, L. Digital instability of a confined elastic meniscus. Proc. Natl Acad. Sci. USA 110, 12545–12548 (2013).
Ogden, R. & Roxburgh, D. A pseudo–elastic model for the Mullins effect in filled rubber. Proc. R. Soc. Lond. Ser. A 455, 2861–2877 (1999).
Lin, S., Zhou, Y. & Zhao, X. Designing extremely resilient and tough hydrogels via delayed dissipation. Extreme Mech. Lett. 1, 70–75 (2014).
Hong, S. et al. 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv. Mater. 27, 4035–4040 (2015).
Darnell, M. C. et al. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials 34, 8042–8048 (2013).
Nemir, S., Hayenga, H. N. & West, J. L. PEGDA hydrogels with patterned elasticity: Novel tools for the study of cell response to substrate rigidity. Biotechnol. Bioeng. 105, 636–644 (2010).
Dugas, V. & Chevalier, Y. Surface hydroxylation and silane grafting on fumed and thermal silica. J. Colloid Interface Sci. 264, 354–361 (2003).
Yoshida, W., Castro, R. P., Jou, J.-D. & Cohen, Y. Multilayer alkoxysilane silylation of oxide surfaces. Langmuir 17, 5882–5888 (2001).
Muir, B. V., Myung, D., Knoll, W. & Frank, C. W. Grafting of cross-linked hydrogel networks to titanium surfaces. ACS Appl. Mater. Interfaces 6, 958–966 (2014).
Cha, C. et al. Tailoring hydrogel adhesion to polydimethylsiloxane substrates using polysaccharide glue. Angew. Chem. Int. Ed. 52, 6949–6952 (2013).
Stile, R. A., Barber, T. A., Castner, D. G. & Healy, K. E. Sequential robust design methodology and X-ray photoelectron spectroscopy to analyze the grafting of hyaluronic acid to glass substrates. J. Biomed. Mater. Res. 61, 391–398 (2002).
Bai, Y. et al. Transparent hydrogel with enhanced water retention capacity by introducing highly hydratable salt. Appl. Phys. Lett. 105, 151903 (2014).
Yang, C. H. et al. Ionic cable. Extreme Mech. Lett. 3, 59–65 (2015).
Acknowledgements
The authors thank A. Wang and L. Griffith for their help on the cell viability test. This work is supported by ONR (No. N00014-14-1-0528), MIT Institute for Soldier Nanotechnologies and NSF (No. CMMI-1253495). H.Y. acknowledges the financial support from Samsung Scholarship. X.Z. acknowledges the supports from NIH (No. UH3TR000505) and MIT Materials Research Science and Engineering Center.
Author information
Authors and Affiliations
Contributions
X.Z. and H.Y. conceived the idea. H.Y., T.Z., S.L., G.A.P. and X.Z. designed the research. H.Y., S.L. and G.A.P. carried out the experiments and T.Z. performed the numerical simulation. H.Y., T.Z., S.L., G.A.P. and X.Z. analysed and interpreted the results. X.Z. drafted the manuscript and all authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2915 kb)
Supplementary Movie 1
Supplementary Movie 1 (MOV 3536 kb)
Supplementary Movie 2
Supplementary Movie 2 (MOV 3514 kb)
Supplementary Movie 3
Supplementary Movie 3 (MOV 3643 kb)
Supplementary Movie 4
Supplementary Movie 4 (MOV 18689 kb)
Supplementary Movie 5
Supplementary Movie 5 (MOV 28212 kb)
Supplementary Movie 6
Supplementary Movie 6 (MOV 17095 kb)
Supplementary Movie 7
Supplementary Movie 7 (MOV 5154 kb)
Supplementary Movie 8
Supplementary Movie 8 (MOV 2085 kb)
Supplementary Movie 9
Supplementary Movie 9 (MOV 1602 kb)
Rights and permissions
About this article
Cite this article
Yuk, H., Zhang, T., Lin, S. et al. Tough bonding of hydrogels to diverse non-porous surfaces. Nature Mater 15, 190–196 (2016). https://doi.org/10.1038/nmat4463
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4463
This article is cited by
-
Reusable dismantlable adhesion interfaces induced by photodimerization and thermo/photocleavage reactions
Polymer Journal (2024)
-
Surface hydrophobization of hydrogels via interface dynamics-induced network reconfiguration
Nature Communications (2024)
-
A 3D printable tissue adhesive
Nature Communications (2024)
-
Accelerated intestinal wound healing via dual electrostimulation from a soft and biodegradable electronic bandage
Nature Electronics (2024)
-
Organohydrogel-based transparent terahertz absorber via ionic conduction loss
Nature Communications (2024)