Abstract
Treatment with ionizing radiation (IR) can lead to the accumulation of tumor-infiltrating regulatory T cells (Treg cells) and subsequent resistance of tumors to radiotherapy. Here we focused on the contribution of the epidermal mononuclear phagocytes Langerhans cells (LCs) to this phenomenon because of their ability to resist depletion by high-dose IR. We found that LCs resisted apoptosis and rapidly repaired DNA damage after exposure to IR. In particular, we found that the cyclin-dependent kinase inhibitor CDKN1A (p21) was overexpressed in LCs and that Cdkn1a−/− LCs underwent apoptosis and accumulated DNA damage following IR treatment. Wild-type LCs upregulated major histocompatibility complex class II molecules, migrated to the draining lymph nodes and induced an increase in Treg cell numbers upon exposure to IR, but Cdkn1a−/− LCs did not. Our findings suggest a means for manipulating the resistance of LCs to IR to enhance the response of cutaneous tumors to radiotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Blanpain, C., Mohrin, M., Sotiropoulou, P.A. & Passegue, E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell 8, 16–29 (2011).
Liauw, S.L., Connell, P.P. & Weichselbaum, R.R. New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Sci. Transl. Med. 5, 173sr172 (2013).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059 (2007).
Liang, H. et al. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J. Immunol. 190, 5874–5881 (2013).
Deng, L. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2015).
Lee, Y. et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114, 589–595 (2009).
Pardoll, D.M. Spinning molecular immunology into successful immunotherapy. Nat. Rev. Immunol. 2, 227–238 (2002).
Topalian, S.L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Bos, P.D., Plitas, G., Rudra, D., Lee, S.Y. & Rudensky, A.Y. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J. Exp. Med. 210, 2435–2466 (2013).
Chen, D.S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Guilliams, M. et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 14, 571–578 (2014).
Merad, M., Ginhoux, F. & Collin, M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 8, 935–947 (2008).
Merad, M. et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3, 1135–1141 (2002).
Chakraverty, R. & Sykes, M. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood 110, 9–17 (2007).
Hashimoto, D., Miller, J. & Merad, M. Dendritic cell and macrophage heterogeneity in vivo. Immunity 35, 323–335 (2011).
Cummings, R.J. et al. Exposure to Ionizing Radiation Induces the Migration of Cutaneous Dendritic Cells by a CCR7-Dependent Mechanism. J. Immunol. 189, 4247–4257 (2012).
Cummings, R.J., Mitra, S., Foster, T.H. & Lord, E.M. Migration of skin dendritic cells in response to ionizing radiation exposure. Radiat. Res. 171, 687–697 (2009).
Ohl, L. et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004).
Kuo, L.J. & Yang, L.-X. γ-H2AX - A novel biomarker for DNA double-strand breaks. In Vivo 22, 305–309 (2008).
Olive, P.L. & Banath, J.P. The comet assay: a method to measure DNA damage in individual cells. Nat. Protoc. 1, 23–29 (2006).
Muslimovic, A., Ismail, I.H., Gao, Y. & Hammarsten, O. An optimized method for measurement of gamma-H2AX in blood mononuclear and cultured cells. Nat. Protoc. 3, 1187–1193 (2008).
Schwarz, A. et al. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. J. Exp. Med. 201, 173–179 (2005).
Abbas, T. & Dutta, A. p21 in cancer: intricate networks and multiple activities. Nat. Rev. Cancer 9, 400–414 (2009).
Ahrendt, S.A. et al. p21WAF1 expression is associated with improved survival after adjuvant chemoradiation for pancreatic cancer. Surgery 128, 520–530 (2000).
Balomenos, D. et al. The cell cycle inhibitor p21 controls T-cell proliferation and sex-linked lupus development. Nat. Med. 6, 171–176 (2000).
Perucca, P. et al. Loss of p21 CDKN1A impairs entry to quiescence and activates a DNA damage response in normal fibroblasts induced to quiescence. Cell Cycle 8, 105–114 (2009).
Seré, K. et al. Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity 37, 905–916 (2012).
Fei, P. & El-Deiry, W.S. P53 and radiation responses. Oncogene 22, 5774–5783 (2003).
Dejean, A.S. et al. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat. Immunol. 10, 504–513 (2009).
Wang, Q. et al. The ataxia telangiectasia mutated kinase pathway regulates IL-23 expression by human dendritic cells. J. Immunol. 190, 3246–3255 (2013).
Idoyaga, J. et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J. Clin. Invest. 123, 844–854 (2013).
Dotto, G.P. p21(WAF1/Cip1): more than a break to the cell cycle? Biochimica Biophysica Acta Rev. Cancer 1471, M43–M56 (2000).
Gartel, A.L. & Tyner, A.L. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol. Cancer Ther. 1, 639–649 (2002).
Wouters, B.G., Giaccia, A.J., Denko, N.C. & Brown, J.M. Loss of p21Waf1/Cip1 sensitizes tumors to radiation by an apoptosis-independent mechanism. Cancer Res. 57, 4703–4706 (1997).
Allan, L.A. & Fried, M. p53-dependent apoptosis or growth arrest induced by different forms of radiation in U2OS cells: p21WAF1/CIP1 repression in UV induced apoptosis. Oncogene 18, 5403–5412 (1999).
Wouters, B.G., Denko, N.C., Giaccia, A.J. & Brown, J.M. A p53 and apoptotic independent role for p21waf1 in tumour response to radiation therapy. Oncogene 18, 6540–6545 (1999).
Hon, H., Rucker, E.B. III, Hennighausen, L. & Jacob, J. bcl-xL is critical for dendritic cell survival in vivo. J. Immunol. 173, 4425–4432 (2004).
Modi, B.G. et al. Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science 335, 104–108 (2012).
Schwarz, A. et al. Langerhans cells are required for UVR-induced immunosuppression. J. Invest. Dermatol. 130, 1419–1427 (2010).
Lawrence, T.S., Blackstock, A.W. & McGinn, C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin. Radiat. Oncol. 13, 13–21 (2003).
Kachikwu, E.L. et al. Radiation enhances regulatory T cell representation. Int. J. Radiat. Oncol. Biol. Phys. 81, 1128–1135 (2011).
Formenti, S.C. & Demaria, S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J. Natl. Cancer Inst. 105, 256–265 (2013).
Begg, A.C., Stewart, F.A. & Vens, C. Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer 11, 239–253 (2011).
von Boehmer, H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 6, 338–344 (2005).
Burnette, B.C. et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 71, 2488–2496 (2011).
Klug, F. et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24, 589–602 (2013).
Ginhoux, F. et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 206, 3115–3130 (2009).
Overwijk, W.W.R.N. B16 as a mouse model for human melanoma. Current Protocols in Immunology 2001 (May, Chapter 20, Unit 20).
Miller, J.C. et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 13, 888–899 (2012).
Duez, P., Dehon, G., Kumps, A. & Dubois, J. Statistics of the Comet assay: a key to discriminate between genotoxic effects. Mutagenesis 18, 159–166 (2003).
Acknowledgements
We thank J. Manfredi and S. Aronson (Icahn School of Medicine at Mount Sinai) for Trp53−/− mice; P. Heeger (Icahn School of Medicine at Mount Sinai) for FoxP3-DTR mice; M.C. Nussenzweig (The Rockefeller University) for Ly75−/− mice; S. Ghaffari (Icahn School of Medicine at Mount Sinai) for Foxo3−/− mice; C. Rivera for help in maintaining mouse colonies; R. Bagnell (University of North Carolina) for the Image-J CometScore module; A. Nussenzweig for input on the manuscript; the Flow Cytometry, Microscopy, and Irradiator Shared Resource Facilities of the Icahn School of Medicine at Mount Sinai for contributions; and the Immunological Genome Project Consortium for resources. Supported by the US National Institutes of Health (T32 CA078207-14 and T32 GM007280 to J.G.P.), the American Medical Association (J.G.P.), the National Institute of Arthritis, Musculoskeletal and Skin Diseases of the US National Institutes of Health (R00 AR062595 to J.I.) and the National Cancer Institute of the US National Institutes of Health (R01 CA173861 and R01 CA154947 to M.M.).
Author information
Authors and Affiliations
Contributions
J.G.P., J.I. and M.M. contributed to the design of and discussions of the work; J.G.P. and J.I. performed experiments, analyzed data and prepared the figures; H.S. contributed to in vivo tumor experiments; B.H. contributed to RT-PCR experiments; C.L.B. and S.G. developed protocols for the comet assay and provided Foxo3−/− mice; M.L. contributed to the maintenance of animal colonies and generation of BM chimeras; J.G.P., J.I. and M.M. wrote the manuscript; and all authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 LCs are resistant to sub-lethal (6 Gy) and lethal (12 Gy) doses of IR.
(a) CD45.1 mice were exposed to either 6 Gy (sub-lethal) or 12 Gy (lethal) IR. In the case of lethal irradiation, mice were injected with BM from CD45.2 mice to avoid IR-induced death. Kinetics of the absolute number of CD45.1 epidermal LC in the ears skin is shown relative to the absolute number of cells before irradiation. (b) Scatter plot of γ-H2AX in epidermal LCs as assessed by flow cytometry 24 h following exposure to either 6 Gy or 12 Gy IR-treatment. (c) Percentage of DNA damage measured by the COMET assay in skin epidermal LCs 24 h after by either 6 Gy and 12 Gy IR-treatment. For (a – b) data are pooled from two independent experiment, n=5 mice total. Data are shown as mean +/− SD. For (c) data are representative of 2 independent experiments with at least n=50 COMETs counted per experiment, and is shown as a whisker plot depicting the 10th, 50th, and 90th percentiles with outliers.
Supplementary Figure 2 Ccr7−/− LCs are a phenocopy of wild-type LCs in DNA-repair kinetics after exposure to IR.
(a) Representative bar graph of fold induction of γ-H2AX over baseline values in Ccr7−/− LCs. LC cell suspension were irradiated with 6 Gy and analyzed for γ-H2AX by flow cytometry 15 min and 2 h later. In the case of 24 h time-point, whole mice were irradiated and LCs subsequently harvested by enzymatic digestion for γ-H2AX analysis by flow cytometry. (b) Representative whisker plot of % DNA damage measured by COMET assay of Ccr7−/− LC isolated by flow cytometry 24 h after exposure to 6 Gy IR. (c) Bar graph shows the fold induction of γ-H2AX in epidermal LCs as assessed by flow cytometry 24 h following exposure to either 6 Gy IR-, UV- or Cisplatin- treatment. For (a & c) n=3 mice/group, data representative of 2 independent experiments and shown mean +/− SD. For (b) data are representative of 2 independent experiments with at least n=50 COMETs counted per experiment, and is shown as a whisker plot depicting the 10th, 50th, and 90th percentiles with outliers. *p<0.05, **p≤0.01 and ***p≤0.001.
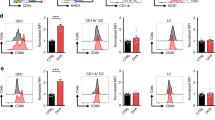
Supplementary Figure 3 Cdkn1a expression in mutant mice and absence of DNA-repair defects in alternative pathways.
(a) WT, Cdkn1a−/−, Trp53−/− and Nfe2l2−/− mice were treated with 6 Gy IR. 24 h after irradiation, LCs were flow-purified from the skin and Cdkn1a levels were assessed by QPCR. Mean +/− SD of two experiments with a total of 4 mice is shown. (b) As in a, but CDKN1A protein levels were measured 48 h after IR by flow cytometry. Shown is a representative experiment of two, and numbers represent the MFI. (c) WT, Cdkn1a−/−, ATM−/− and Foxo3−/− mice were exposed to 6 Gy IR. 24 h after IR, expression of γ-H2AX was assessed by flow cytometry, and is expressed as the mean +/− SD fold-induction relative to the non-irradiated control (n= 4-8 mice in 2-3 different experiments). p values of <.05 are labeled as * and p≤0.01 are labeled as **.
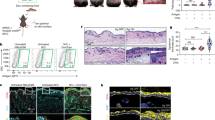
Supplementary Figure 4 IR-treated mice are more susceptible to tumor growth shortly after irradiation.
(a) Mice were irradiated with 6 Gy followed 2 h later by the injection of 1x106 EL-4 tumor cells subcutaneously, and tumor volume was assessed at the indicated time points. (b) Bar graph of tumor (left) and tdLN (right) infiltrating Foxp3+ Treg cells as assessed 12 d after tumor challenge. (c) Mice were exposed to 6 Gy IR or left untreated. 12-24 h or 5 weeks after IR-treatment, mice were inoculated subcutaneously with 5×105 B16 melanoma cell line. Tumor volume was assessed at the indicated time points. (d) Tumor infiltrating (left) and tdLN (right) Foxp3+ Treg cells was evaluated 12 d after tumor challenge. In all cases, data show the mean +/− SD of 5-8 animals in 1-2 experiments. p values of ≤0.01 are labeled as ** and p≤0.001 as ***.
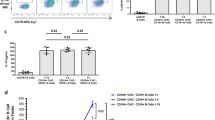
Supplementary Figure 5 LCs from chimeric mice lack CDKN1A but express DEC205.
(a) WT mice were treated with 12 Gy IR and transplanted with BM from Ly75−/− mice. Two months after BM transplantation, the expression of DEC205 was evaluated by flow cytometry. Representative gating strategy demonstrating that the only cells expressing DEC205 in the sdLN of BM chimeras are Epcam+CD11b+MHCII+ embryonic-derived LCs. The flow plot is pre-gated on all sdLN APCs (CD11c+MHCII+). (b) CD45.2 WT or Cdkn1a−/− mice were treated with 12 Gy IR and transplanted with BM from CD45.1 mice. LCs were analyzed 9-12 weeks after transplantation by flow cytometry for the expression of CD45.1 donor marker. (c) As in (b), but LCs were flow-purified and analyzed for the expression of Cdkn1a by QPCR. For (a, b) Data are representative of n=10 mice. For (c) data is representative of one of two independent experiments with 2-3 animals per group.
Supplementary Figure 6 MHC class II expression in LCs is required for the generation of Treg cells after IR treatment.
(a,b) Foxp3-DTR mice were exposed to 6 Gy IR followed by the subcutaneous inoculation of 5 x 105 B16 tumor cells 12 h later. 5 d after tumor challenge, and every 2 d thereafter, mice were inoculated with DT i.p. (a) Tumor volumes were assessed at the indicated time points. (b) Tumor infiltrating and tdLN Foxp3+ Treg cells were assessed 12 d after tumor challenge by flow cytometry. Shown is the mean +/− SD of 3 animals in one experiment. (c, d) H2−/− mice were lethally irradiated and reconstituted with BM cells isolated from either WT or Cdkn1a−/− mice. Two months post-transplantation, mice were further 6 Gy IR-treated and injected subcutaneously with 5 x 105 B16 melanoma cells 12 h later. (c) Graph depicting tumor volumes as the mean +/− SD of n=4 mice in two different experiments. (d) Tumor infiltrating Treg cells are shown as the percentage of total hematopoietic cells (gated as CD45+ cells) in B16 tumors at 14 d post-B16 injection. Data are pooled from two independent experiments, n≥2 mice per experiment. Data are plotted as Mean +/− SD. p values of ≤0.01 are labeled as ** and p≤0.001 as ***.
Supplementary information
Supplementary Text and Figures
Figures 1–6 (PDF 861 kb)
Supplementary Table 1
List of antibodies for flow cytometry (PDF 98 kb)
Rights and permissions
About this article
Cite this article
Price, J., Idoyaga, J., Salmon, H. et al. CDKN1A regulates Langerhans cell survival and promotes Treg cell generation upon exposure to ionizing irradiation. Nat Immunol 16, 1060–1068 (2015). https://doi.org/10.1038/ni.3270
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3270
This article is cited by
-
Microarray analysis identifies coding and non-coding RNA markers of liver injury in whole body irradiated mice
Scientific Reports (2023)
-
Orchestration of myeloid-derived suppressor cells in the tumor microenvironment by ubiquitous cellular protein TCTP released by tumor cells
Nature Immunology (2021)
-
The inflammatory pathogenesis of colorectal cancer
Nature Reviews Immunology (2021)
-
Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses
Cellular & Molecular Immunology (2020)
-
Regulating colonic dendritic cells by commensal glycosylated large surface layer protein A to sustain gut homeostasis against pathogenic inflammation
Mucosal Immunology (2020)