Abstract
Stem cells, including cancer stem cells (CSCs), require niches to maintain stemness, yet it is unclear how CSCs maintain stemness in the suboptimal environment outside their niches during invasion. Postnatal co-deletion of Pten and Trp53 in mouse neural stem cells (NSCs) leads to the expansion of these cells in their subventricular zone (SVZ) niches but fails to maintain stemness outside the SVZ. We discovered that Qki is a major regulator of NSC stemness. Qk deletion on a Pten−/−; Trp53−/− background helps NSCs maintain their stemness outside the SVZ in Nes-CreERT2; QkL/L; PtenL/L; Trp53L/L mice, which develop glioblastoma with a penetrance of 92% and a median survival time of 105 d. Mechanistically, Qk deletion decreases endolysosome-mediated degradation and enriches receptors essential for maintaining self-renewal on the cytoplasmic membrane to cope with low ligand levels outside niches. Thus, downregulation of endolysosome levels by Qki loss helps glioma stem cells (GSCs) maintain their stemness in suboptimal environments outside their niches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
He, S., Nakada, D. & Morrison, S.J. Mechanisms of stem cell self-renewal. Annu. Rev. Cell Dev. Biol. 25, 377–406 (2009).
Ming, G.L. & Song, H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702 (2011).
Suh, H., Deng, W. & Gage, F.H. Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 25, 253–275 (2009).
Fuchs, E., Tumbar, T. & Guasch, G. Socializing with the neighbors: stem cells and their niche. Cell 116, 769–778 (2004).
Scadden, D.T. The stem-cell niche as an entity of action. Nature 441, 1075–1079 (2006).
Calabrese, C. et al. A perivascular niche for brain tumor stem cells. Cancer Cell 11, 69–82 (2007).
Li, Z. et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15, 501–513 (2009).
Lane, S.W., Scadden, D.T. & Gilliland, D.G. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood 114, 1150–1157 (2009).
Merlos-Suárez, A. et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 8, 511–524 (2011).
Li, L. & Neaves, W.B. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 66, 4553–4557 (2006).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Dunn, G.P. et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 26, 756–784 (2012).
Hemmati, H.D. et al. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. USA 100, 15178–15183 (2003).
Rich, J.N. & Eyler, C.E. Cancer stem cells in brain tumor biology. Cold Spring Harb. Symp. Quant. Biol. 73, 411–420 (2008).
Vescovi, A.L., Galli, R. & Reynolds, B.A. Brain tumour stem cells. Nat. Rev. Cancer 6, 425–436 (2006).
Giese, A., Bjerkvig, R., Berens, M.E. & Westphal, M. Cost of migration: invasion of malignant gliomas and implications for treatment. J. Clin. Oncol. 21, 1624–1636 (2003).
Scherer, H.J. The forms of growth in gliomas and their practical significance. Brain 63, 1–35 (1940).
Scherer, H.J. Structural development in gliomas. Am. J. Cancer 34, 333–351 (1938).
Hu, J. et al. Neutralization of terminal differentiation in gliomagenesis. Proc. Natl. Acad. Sci. USA 110, 14520–14527 (2013).
Chen, A.J. et al. STAR RNA-binding protein Quaking suppresses cancer via stabilization of specific miRNA. Genes Dev. 26, 1459–1472 (2012).
Wang, Y., Vogel, G., Yu, Z. & Richard, S. The QKI-5 and QKI-6 RNA binding proteins regulate the expression of microRNA 7 in glial cells. Mol. Cell. Biol. 33, 1233–1243 (2013).
Conn, S.J. et al. The RNA binding protein Quaking regulates formation of circRNAs. Cell 160, 1125–1134 (2015).
Darbelli, L. & Richard, S. Emerging functions of the Quaking RNA-binding proteins and link to human diseases. Wiley Interdiscip. Rev. RNA 7, 399–412 (2016).
Muzumdar, M.D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Imayoshi, I. et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 11, 1153–1161 (2008).
Holland, E.C. et al. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat. Genet. 25, 55–57 (2000).
Alcantara Llaguno, S. et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 15, 45–56 (2009).
Jacques, T.S. et al. Combinations of genetic mutations in the adult neural stem cell compartment determine brain tumour phenotypes. EMBO J. 29, 222–235 (2010).
Verhaak, R.G. et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110 (2010).
Hafner, M. et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141, 129–141 (2010).
Lathia, J.D., Mack, S.C., Mulkearns-Hubert, E.E., Valentim, C.L. & Rich, J.N. Cancer stem cells in glioblastoma. Genes Dev. 29, 1203–1217 (2015).
Chen, J., McKay, R.M. & Parada, L.F. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell 149, 36–47 (2012).
Zheng, H. et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature 455, 1129–1133 (2008).
Mellman, I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12, 575–625 (1996).
Levkowitz, G. et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell 4, 1029–1040 (1999).
Levkowitz, G. et al. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 12, 3663–3674 (1998).
Ying, H. et al. Mig-6 controls EGFR trafficking and suppresses gliomagenesis. Proc. Natl. Acad. Sci. USA 107, 6912–6917 (2010).
Chen, Y., Tian, D., Ku, L., Osterhout, D.J. & Feng, Y. The selective RNA-binding protein Quaking I (QKI) is necessary and sufficient for promoting oligodendroglia differentiation. J. Biol. Chem. 282, 23553–23560 (2007).
Chénard, C.A. & Richard, S. New implications for the QUAKING RNA binding protein in human disease. J. Neurosci. Res. 86, 233–242 (2008).
Brennan, C.W. et al. The somatic genomic landscape of glioblastoma. Cell 155, 462–477 (2013).
Qaddoumi, I. et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 131, 833–845 (2016).
Bandopadhayay, P. et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat. Genet. 48, 273–282 (2016).
Bian, Y. et al. Downregulation of tumor suppressor QKI in gastric cancer and its implication in cancer prognosis. Biochem. Biophys. Res. Commun. 422, 187–193 (2012).
Yu, F. et al. Post-transcriptional repression of FOXO1 by QKI results in low levels of FOXO1 expression in breast cancer cells. Oncol. Rep. 31, 1459–1465 (2014).
Yang, G. et al. RNA-binding protein Quaking, a critical regulator of colon epithelial differentiation and a suppressor of colon cancer. Gastroenterology 138, 231–240.e1, 5 (2010).
Zhao, Y. et al. The tumor suppressing effects of QKI-5 in prostate cancer: a novel diagnostic and prognostic protein. Cancer Biol. Ther. 15, 108–118 (2014).
Lu, W. et al. QKI impairs self-renewal and tumorigenicity of oral cancer cells via repression of SOX2. Cancer Biol. Ther. 15, 1174–1184 (2014).
Aguirre, A.J. et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 17, 3112–3126 (2003).
Bhat, K.P. et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell 24, 331–346 (2013).
Acknowledgements
We thank S. Jiang, Z. Fang, and K. Zhao for mouse husbandry and care, and all members of the Hu laboratory for helpful discussions. We thank Y. Qi for performing alternative splicing analyses. We thank K. Ryszard Gabrusiewicz for helping us analyze the glioblastoma TMA. We thank K. Dunner for electron microscopy studies. We also thank D.M. Wildrick for editorial assistance. J.H. is supported by an NIH K99/R00 Pathway to Independence Award (R00 CA172700), a University of Texas Rising STARs award, an NCI Brain Cancer SPORE Career Development Award (2P50CA127001), and a Sidney Kimmel Scholar Award. A.L.H. was supported by the HHMI Medical Research Fellows Program. A.B.H. is supported by NIH R01 CA120813. Y.C. is supported by NCI R00CA175290 and Texas CPRIT grant RR140071.
Author information
Authors and Affiliations
Contributions
J.H. and T.S. designed the study. T.S., A.L.H., L.Y., X.Z., C.D., and B.D.L. performed the experiments. S.Z., Q.W., Y.Z., Y.C., and R.G.W.V. performed bioinformatics analysis. G.N.F. provided pathological analyses for the QPP gliomas. Q.C. helped to analyze TMA samples. J.W.H., Y.Y., B.H., and A.B.H. contributed animal and clinical samples.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Qki is expressed in NSCs and inhibits self-renewal of Pten–/–; Trp53–/– PM-NSCs.
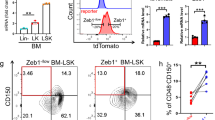
(a) Strategy to identify Qki as a potential tumor suppressor whose deletion might enhance the self-renewal of NSCs. (b) Percentage of Pten–/–; Trp53–/– (PP) PM-NSCs that are able to form secondary spheres when overexpressing Qki-6 or mock (control) (n = 7/group; Student’s t test). (c) Representative immunofluorescence image of co-staining for Qki and nestin in the LV–SVZ and TV–SVZ of P7 mice. (d) Scheme for generating the Qki-loxP conditional knockout allele. (e) Immunoblotting of Qki in NSCs derived from the SVZs of wild-type, Nes-CreERT2; QkL/+, and Nes-CreERT2; QkL/L mice. Vinculin (Vin) is loading control. (f) Representative images of GFP + nestin and GFP + Gfap staining of the SVZ of a P10 Nes-CreERT2 mTmG mouse that was injected with tamoxifen at P8. Scale bars, 100 μm. The experiments shown in b, c, e, and f were replicated three times in the laboratory.
Supplementary Figure 2 Qk deletion increases NSC self-renewal capacity and decreases NSC differentiation.
(a) Representative images and quantification of immunofluorescence staining for short-term BrdU (ST-BrdU) and Qki in P2 SVZs from Nes-CreERT2; Qk+/+ and Nes-CreERT2; QkL/L mice treated with tamoxifen at P0.5 and labeled with BrdU at P1 (Student’s t test; n = 7 for each group). (b) Representative images and quantification of immunofluorescence co-staining for Id1 and Gfap in SVZs from P12 Nes-CreERT2; Qk+/+ and Nes-CreERT2; QkL/L mice injected with tamoxifen at P1 (Student’s t test; n = 3 for each group). (c) The percentage of apoptotic cells is indicated as determined by positive immunofluorescence staining for cleaved (Cle’d) caspase-3 in SVZs from P12 Nes-CreERT2; Qk+/+ and Nes-CreERT2; QkL/L mice in vivo (left) and Qk+/+ and Qk–/– NSCs (passage 5) cultured in vitro (right) (Student’s t test; n = 7 for each group; n.s., not significant). (d) Representative images of Qk-wild-type and Qk-null NSCs at passage 25 stained for nestin and Qki. (e,f) Representative images and quantification of immunohistochemical staining for Mbp and Olig2 in P12 corpus callosum (CC) from Nes-CreERT2; Qk+/+ and Nes-CreERT2; QkL/L mice treated with tamoxifen at P2 (n = 5–6/group; Student’s t test). (g) Representative images and quantification of Tuj1 and Qki immunefluorescent staining in E14.5 cortex of Nes-CreERT2; Qk+/+ and Nes-CreERT2; QkL/L embryos treated with tamoxifen at day E9.5 (n = 5/group; Student’s t test). (h) Representative images and quantification of immunofluorescent staining of Gfap in E17.5 cortex from Nes-CreERT2; Qk+/+ and Nes-CreERT2; QkL/L embryos treated with tamoxifen at day E9.5 (n = 6/group; Student’s t test). Scale bars: 100 μm in a and d–f, and 50 μm in b. Error bars, s.d. CC, corpus callosum; MZ, marginal zone; CP, cortical plate; SP, subplate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone. The experiments shown in all panels were replicated three times in the laboratory.
Supplementary Figure 3 Deletion of Pten and Trp53 expands NSCs in SVZ niches yet fails to maintain NSC stemness outside the niches.
(a) Representative immunofluorescence images and quantification of Ki67 + GFP co-staining or Sox2 + GFP co-staining in the SVZs or thalamus/hypothalamus (TH/HY) of P38 Nes-CreERT2 mTmG and Nes-CreERT2 PP mTmG mice injected at P8 (n = 6/group; Student’s t test). (b) Representative immunofluorescence images of Olig2 + GFP co-staining, NeuN + GFP co-staining, or S100b + GFP co-staining of thalamus/hypothalamus (TH/HY) in P38 Nes-CreERT2 mTmG and Nes-CreERT2 PP mTmG mice injected with tamoxifen at P8. Scale bars, 100 μm. *P < 0.0001; n.s., not significant. Error bars, s.d. The experiments shown in all panels were replicated three times in the laboratory.
Supplementary Figure 4 Qk deletion maintains stemness of PM-NSCs outside of their niches.
(a) Representative H&E images of SCID mouse brains with injection of PP PM-NSCs in the lateral ventricle (LV) and cortex (CTX). (b) Representative immunofluorescence images of Numb staining of SCID mouse brains with injection of PP PM-NSCs in the left lateral ventircle and cortex. (c) Representative immunofluorescence images of Egfr staining of SCID mouse brains with injection of PP PM-NSCs in the left lateral ventricle and cortex. (d) Representative immunofluorescence images of Fgfr staining of SCID mouse brains with injection of PP PM-NSCs in the left lateral ventricle and cortex. (e) Representative immunofluorescence image of Ki67 + Sox2 + GFP co-staining of TH/HY in P38 Nes-CreERT2 QPP mTmG mice that were injected at P8. (f) Representative immunofluorescence images and quantification of Mbp + GFP co-staining of thalamus/hypothalamus (TH/HY) in P38 Nes-CreERT2 PP mTmG and Nes-CreERT2 QPP mTmG mice injected with tamoxifen at P8 (n = 5/group; Student’s t test). (g) Representative immunohistochemistry images of Qki staining of P38 Nes-CreERT2 PP and Nes-CreERT2 QPP mice injected with tamoxifen at P8. (h) Representative immunohistochemistry images of Pten staining in P38 Nes-CreERT2 PP and Nes-CreERT2 QPP mice injected with tamoxifen at P8. (i) Immunoblotting of Qki and Pten in neurosphere cultures derived from the SVZs of Nes-CreERT2; Qk+/+, Nes-CreERT2; QkL/L, Nes-CreERT2 PP, and Nes-CreERT2 QPP mice. (i) PCR of the Trp53 locus in neurosphere cultures derived from the SVZs of Nes-CreERT2; QkL/L, Nes-CreERT2 PP, and Nes-CreERT2 QPP mice. Scale bars: 100 μm in a, b, g, and h, and 50 μm in c–f. Error bars, s.d. The experiments in all panels were replicated three times in the laboratory.
Supplementary Figure 5 Nes-CreERT2; QkL/L; PtenL/L; Trp53L/L (QPP) mTmG gliomas recapitulate human glioblastoma.
(a,b) Representative immunohistochemistry staining of Notch1 (a) and nestin (b) in QPP gliomas. (c) Representative QPP glioma H&E images of a tumor consisting of spindle-shaped cells with oval or round nuclei with dense chromatin (top left); a tumor with abundant multinucleated giant cells (top right); a tumor consisting of cells with eosinophilic fibrillary cytoplasm and round or polygonal nuclei with prominent nucleoli (bottom left); and a tumor with structures reminiscent of Homer–Wright rosettes (bottom right). Scale bars, 100 μm. (d) Kaplan–Meier survival curves (log-rank test) of Nes-CreERT2; QkL/L, Nes-CreERT2; PtenL/L; Trp53L/L (PP), and Nes-CreERT2; QkL/L; PtenL/L; Trp53L/L (QPP) mice treated with tamoxifen at P30. P < 0.0001 means that the differences between Nes-CreERT2; QkL/L; PtenL/L; Trp53L/L and any other cohorts are significant. (e) Kaplan–Meier survival curves (log-rank test) of Nes-CreERT2; PtenL/L; Ink4a/ArfL/L and Nes-CreERT2; QkL/L; PtenL/L; Ink4a/ArfL/L mice treated with tamoxifen at P8.
Supplementary Figure 6 Qki is a new regulator of endolysosomes.
(a) Schematic of stable isotope labeling by amino acids in cell culture (SILAC)-based proteomic analysis. LC–MS/MS, liquid chromatography and tandem mass spectrometry; m/z, mass-to-charge ratio. (b) Top pathways (P < 0.001) that are enriched by Qk deletion as detected by Ingenuity Pathway Analysis (IPA).
Supplementary Figure 7 Qk deletion promotes self-renewal by decreasing endolysosome-dependent receptor degradation.
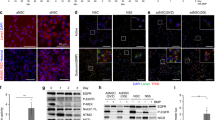
(a) Immunoblotting of QKI and LAMP1 in human GSCs with shRNAs against QKI and control shRNA. (b) Representative images and quantification of immunofluorescent co-staining for long-term BrdU and Lamp1 in the SVZs of P12 Nes-CreERT2; Qk+/+ and Nes-CreERT2; QkL/L mice treated with tamoxifen at P1 (n = 3/group; Student’s t test). (c) Immunoblotting of transferrin receptor (TfR) in Qk+/+ and Qk–/– NSCs and in PP and QPP PM-NSCs. (d) Endocytosis of Egfr was measured by the low-pH-sensitive red EGF probe in Qk+/+ and Qk–/– NSCs and in PP and QPP PM-NSCs after Egf depletion for 1 h (Student’s t test; n = 83 for Qk+/+ NSCs, 93 for Qk–/– NSCs, 89 for PP PM-NSCs, and 110 for QPP PM-NSCs). (e) Expression correlation between QKI and CLTC, RAB5C, RAB28, SNX2, SNX3, and GPHN in the TCGA microarray data set (n = 543; Pearson correlation). (f) Subcutaneous xenograft tumor formation of PP PM-NSCs with Lamp1 knockdown (18 individual animals) or control knockdown (9 individual animals). Tumor volumes (mm3) were calculated using the formula volume = ½(length × width2). (g) Left, immunoblotting of Lamp1, Sox2, and Gfap in QPP PM-NSCs with Tfe3 overexpression or mock (control). Right, percentages of QPP PM-NSCs with Tfe3 overexpression or mock that can form neurospheres from single cells cultured in either full-strength growth factors or a 1:10 dilution of growth factors (Student’s t test, *P < 0.0001). Scale bar: 50 μm in b, and 20 μm in d. Error bars: s.d. in b and g, and s.e.m. in d. The experiments in a, b, d, and g were replicated three times in the laboratory.
Supplementary Figure 8 QKI levels inversely correlate with receptor levels and patient survival.
(a) Immunoblotting of Lamp1, Notch1, and Fgfr in QPP PM-NSCs after overexpression of Qki-6. (b) Immunoblotting of self-renewal and differentiation markers in Pten–/–; Trp53–/– (PP) and Qk–/–; Pten–/–; Trp53–/–(QPP) PM-NSCs treated with EGFR inhibitor. (c) Percentages of PP PM-NSCs treated with 10 μM Notch1 inhibitor (DAPT, BMS-708163, RO4929097), 250 μM Wnt inhibitor (C59), and 1 μM EGFR inhibitor (erlotinib) that can form neurospheres from single cells cultured in either full-strength growth factors (20 ng/ml EGF + 10 ng/ml FGF) or a 1:10 dilution of growth factors (2 ng/ml EGF + 1 ng/ml FGF) (Student’s t test, *P < 0.001, **P < 0.01). (d) Human TMA glioblastoma samples were plotted according to the percentage of QKI+ cells that were detected by immunohistochemistry. (e) Representative immunohistochemistry images of a normal human brain sample and human glioblastoma samples with high and low percentages of QKI+ cells. (f) Human TMA glioblastoma samples were plotted according to the percentage of punctate LAMP1+ cells that were detected by immunohistochemistry. (g) Representative immunohistochemistry images of a normal human brain sample and human glioblastoma samples with high and low percentages of punctate LAMP1+ cells. White scale bars, 100 μm; black scale bars, 50 μm. The experiments in a–c were replicated three times in the laboratory.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8. (PDF 2298 kb)
Supplementary Table 1
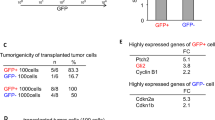
Gene list in both NSCs and PM-NSCs. (XLSX 47 kb)
Supplementary Table 2
Splicing events in both NSCs and PM-NSCs. (XLSX 17 kb)
Supplementary Table 3
SILAC up in Qk null. (XLSX 74 kb)
Supplementary Table 4
SILAC down in Qk null. (XLSX 89 kb)
Supplementary Table 5
Combined RNA–seq SILAC PAR-CLIP. (XLSX 42 kb)
Supplementary Table 6
Immunohistochemistry Qki and punctate Lamp1 with patient survival data. (XLSX 53 kb)
Supplementary Table 7
qPCR primers for verifying Qki targets. (XLSX 48 kb)
Rights and permissions
About this article
Cite this article
Shingu, T., Ho, A., Yuan, L. et al. Qki deficiency maintains stemness of glioma stem cells in suboptimal environment by downregulating endolysosomal degradation. Nat Genet 49, 75–86 (2017). https://doi.org/10.1038/ng.3711
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3711
This article is cited by
-
Balancing lysosome abundance in health and disease
Nature Cell Biology (2023)
-
Kunitz-type protease inhibitor TFPI2 remodels stemness and immunosuppressive tumor microenvironment in glioblastoma
Nature Immunology (2023)
-
DNMT1 Mediated CAHM Repression Promotes Glioma Invasion via SPAK/JNK Pathway
Cellular and Molecular Neurobiology (2022)
-
Qki activates Srebp2-mediated cholesterol biosynthesis for maintenance of eye lens transparency
Nature Communications (2021)
-
Transcriptional profiling of multiple system atrophy cerebellar tissue highlights differences between the parkinsonian and cerebellar sub-types of the disease
Acta Neuropathologica Communications (2020)