Abstract
The pericardium, myocardium, coronary arteries and pulmonary arteries are the main targets for cardiac disease in people who are infected with HIV. Geography and access to highly active anti-retroviral therapy (HAART) have a major influence on which of these targets is affected. In sub-Saharan Africa, where tuberculosis is endemic and access to HAART is limited, the dominant forms of HIV-associated heart disease are pericardial tuberculosis and cardiomyopathy. However, in industrialized countries, where tuberculosis is rare and HAART is widely available, coronary artery disease is the main cause of death and disability in these patients. Observational data suggest that HAART, by preserving immune function, reduces the incidence of myopericardial disease and pulmonary hypertension. The result has been that, although optimal strategies to reduce vascular disease in this population continue to be sought and debated in industrialized nations, the focus of prevention and treatment strategies for HIV-related heart disease in developing countries has been to support the active campaigns to get universal access to HAART in the first place. Herein, we review the cardiac manifestations of HIV in sub-Saharan Africa.
Key Points
-
Studies from Africa confirm that cardiac abnormalities are more common in people infected with HIV compared to normal controls
-
Approximately half of hospitalized patients with HIV, as well as a significant proportion of patients followed up over several years, develop cardiac abnormalities
-
The most common HIV-related cardiac abnormalities are pericardial disease and cardiomyopathy
-
Echocardiography is indicated in HIV-positive patients with cardiac symptoms
-
Tuberculosis is the major cause of HIV-associated pericardial effusion in Africa
-
If pericardial disease or cardiomyopathy is identified, further investigation must be considered to exclude potentially treatable, opportunistic infections
Similar content being viewed by others
Introduction
The link between HIV infection and heart disease was established early in the history of the AIDS pandemic.1 A number of studies have been conducted to determine the spectrum of cardiac pathology associated with HIV and AIDS. These studies show that in Africans with AIDS, the most common cardiac pathology involves either the pericardium or the myocardium; the prevalence for these abnormalities is up to 60%, and patients are usually asymptomatic.2 The prevalence of these abnormalities in the developed world before the widespread use of highly active anti-retroviral therapy (HAART) was approximately 40%.3 This pathology is most frequent in patients with advanced AIDS, where it increases the relative risk of mortality and shortens longevity compared to control patients with matched CD4+ cell counts. HAART does not seem to alter the long-term outcome in patients with established myocardial and pericardial disease.4 However, the introduction of HAART, before the onset of cardiac disease, does seem to protect patients from developing these complications in the first place.4
The three factors that influence the frequency and pattern of cardiac manifestations in people infected with HIV are geography, access to HAART and the degree of immunosuppression.5 Susceptibility to cardiac infections and the spectrum of opportunistic infections involved vary considerably depending on these factors. Evidence also indicates that some anti-retroviral drugs are associated with an increased risk of coronary artery disease.6
The most common manifestations of HIV-associated heart disease in sub-Saharan Africa are pericarditis, cardiomyopathy and pulmonary hypertension. Coronary artery disease, lipodystrophy and metabolic syndrome, although all common in developed countries, are not significant clinical problems in Africa. Although valvular heart disease, as a result of rheumatic heart disease, remains prevalent in Africa,7 no evidence exists of an increase in cases of infective endocarditis associated with HIV infection, as has been described in North America, Spain and France.2,5,8 The virtual absence of HIV-associated endocarditis in Africa might be related to the low prevalence of intravenous drug use among the people who have HIV on that continent, as opposed their counterparts in wealthier nations.
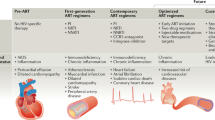
This Review focuses on the cardiac manifestations of HIV in sub-Saharan Africa, where approximately 70% of the 34 million people worldwide with HIV and AIDS live. Sub-Saharan Africa is also where access to HAART is limited to less than 20% of those who need it.9 The 'two faces' of AIDS-associated cardiac disease will be explored; in Africa, opportunistic, infection-related myopericardial disease predominates and access to HAART is limited, and in the developed world, atherosclerosis-associated cardiovascular disease predominates and access to HAART is widespread (Table 1).
Pericarditis
Prevalence and etiology
In sub-Saharan Africa, pericardial disease is often the first manifestation of HIV-related or AIDS-related cardiac disease (Figure 1).2,5 Although anecdotal experience suggest that patients with advanced immunosuppression are more susceptible to pericarditis, this condition may present at any stage of HIV infection. Mycobacterium tuberculosis, a treatable microorganism, is the underlying cause in up to 70% of cases of pericardial effusion.10 Although malignancies such as non-Hodgkin lymphoma and Kaposi sarcoma are important potential causes of pericarditis in the developed world, the published and anecdotal clinical experience from Africa indicates that these malignances rarely cause pericarditis in patients infected with HIV.10,11,12,13 In low-tuberculosis and/or low-HIV prevalence regions, where pericardial effusion is often an incidental finding in asymptomatic patients, pericarditis is usually of idiopathic origin.14 The etiology of large pericardial effusion in symptomatic patients should be investigated thoroughly, as it is often treatable. These investigations should include the use of pericardiocentesis if noninvasive tests are unhelpful.10,14,15
Clinical presentation and diagnostic considerations
Some data indicate that HIV might modify the clinical presentation of pericardial effusion.7 Tuberculous pericarditis in individuals with HIV might arise from dissemination of tuberculosis in a high proportion of patients.16 Up to 33% of patients with HIV who presented to San Francisco General Hospital with symptomatic effusion had evidence of pericardial tamponade, compared with 20% in a non-HIV cohort.17,18 In the Investigation of the Management of Pericarditis in Africa (IMPI Africa) registry, conducted in South Africa, Nigeria and Cameroon, a higher proportion of patients with HIV, compared to those without HIV, presented with dyspnea and evidence of hemodynamic instability.19 Data from Burkina Faso suggested that as many as 40% of patients with HIV might have coexisting left ventricular dysfunction when tuberculosis is the etiology.20 This finding is consistent with the increased rate of electrocardiographic abnormalities, which are suggestive of myopericarditis, observed in the IMPI Africa registry.7,21 We have also shown that HIV infection might be associated with a decreased incidence of constriction in patients with pericardial tuberculosis.22
Diagnosis
A rapid, accurate and simple method to establish the etiology of pericarditis in patients infected with HIV remains a major problem in much of the developing world.23,24 The facilities needed to perform safe pericardiocentesis and pericardial biopsy, which increase the capacity to make a definitive etiological diagnosis in 70–80% of cases,10,25 are lacking, and culture for M. tuberculosis can take up to 8 weeks. As a result, empiric treatment with antituberculosis therapy (usually a combination of pyrazinamide, ethambutol, rifampicin and isoniazid) is the order of the day, with morbid implications for those with alternative diagnoses, such as purulent pericarditis.21
Prognosis and treatment
For patients with AIDS who are not on anti-retroviral therapy, the prognostic implication of a diagnosis of pericardial disease is dire, regardless of geography. In developed countries, the presence of a pericardial effusion conferred a relative risk for mortality of 2.2 compared with control patients matched for CD4+ cell counts.26 To date, no data on the prevalence, prognosis and etiology of pericardial effusions in patients on HAART has been reported. An interesting observation is that as more patients at high risk for tuberculosis use HAART, pericarditis related to immune reconstitution disease might become a growing source of concern.27,28 For those patients in sub-Saharan Africa with a diagnosis of tuberculous pericarditis, improved treatment strategies need to be investigated and implemented, as the current mortality rates of 17–40% are unacceptably high.23,24 The use of adjunctive corticosteroids remains controversial29,30 because the evidence for their effectiveness is inconclusive. To date, only one randomized study, of 58 patients in Zimbabwe, has been specifically designed to test the effectiveness of steroids in patients infected with HIV who have tuberculous pericarditis.31 Despite the favorable effect of steroids on mortality found in this trial, concerns about increased susceptibility to opportunistic illness in immunosuppressed patients are prevalent among practitioners.32 While antituberculosis drugs are the mainstay of therapy, the timing of the introduction and co-administration of HAART is not clear. Concerns about drug interactions and the potentially deleterious effects of immune reconstitution syndrome with early introduction remain,33 and a consensus approach has not been reached.
Cardiomyopathy
Prevalence
Echocardiography and autopsy studies have reported a wide variation in both the prevalence and incidence of cardiomyopathy in people infected with HIV.5 Reported figures depend on the criteria used to define cardiomyopathy, when the studies were performed relative to the availability of HAART, and whether or not the study was on ambulant or hospitalized patients.2 Cross-sectional echocardiography studies from Africa suggest a prevalence of cardiomyopathy of up to 57% in hospitalized patients.20,34,35,36 The only prospective study of ambulant patients from central Africa reported an incidence of 16.9% over 18 months.37 Before the introduction of HAART, data from the developed world suggested a prevalence of 30–40%, and an annual incidence of 15.9 per 1,000 patients per year.3 Both sets of data indicate that, in the absence of HAART, the prevalence and incidence of heart muscle disease is high.
The proportion of newly diagnosed cardiomyopathy in African patients infected with HIV is not known. A study from the US on the underlying causes of cardiomyopathy suggested that HIV is the cause in approximately 4% of cases.38 The inherent limitations of selection and sampling have been a problem for many of these studies, and accurate data from areas of high HIV prevalence is essential for elucidating the epidemiology of this important cardiac complication.
Clinical presentation
In Africa, HIV-associated cardiomyopathy presents in three different clinical settings. The first manifestation is left ventricular abnormality in asymptomatic patients undergoing routine echocardiography (subclinical cardiac dysfunction); the natural history and long-term effects of asymptomatic left ventricular abnormalities on survival in people infected with HIV remain to be fully defined. The range of abnormalities include nondilated left ventricle with impaired systolic function, dilated cardiac chambers with normal ejection fraction, nondilated ventricles with evidence of diastolic dysfunction, and dilated ventricles with impaired systolic function.34,39,40,41 Results from autopsy studies of patients infected with HIV who died from AIDS-related illnesses other than heart failure in the era before the introduction of HAART show similar findings.42,43,44,45,46,47 Viral, protozoan, bacterial, fungal and mycobacterial opportunistic pathogens have also been found in the myocardium at autopsy.48,49
The second setting of HIV-associated cardiomyopathy is in symptomatic patients with signs of heart failure and evidence of dilated ventricles with depressed systolic function. In Africa, the likelihood of developing HIV-associated cardiomyopathy increases with the progression of immunosuppression, which is independently associated with death.2,5 Studies from Cameroon and Rwanda support data from Scotland, which show that symptomatic cardiomyopathy is strongly associated with a CD4+ cell count less than 100 cells/µl.35,36,50
The third setting of HIV-associated cardiomyopathy is in hospitalized patients with advanced AIDS-related opportunistic illnesses, who develop heart failure in the absence of prior evidence of cardiac disease. Of 157 consecutive patients admitted to a referral hospital in Zimbabwe, 30% were found to have impaired left ventricular systolic function.34 Cytokines (e.g. tumor necrosis factor) are expressed in abundance in sick patients with AIDS, and are capable of upregulating the expression of inducible nitric oxide, which increases the production of reactive oxygen species and interferes with calcium homeostasis and myocyte contractility.51 Autonomic dysregulation might also lead to transient left ventricular dysfunction in these patients.52
Etiology and pathogenesis
Unfortunately, there is a paucity of published work on the pathogenesis and etiology of HIV-associated cardiomyopathy in Africa. Whereas viral myocarditis has been suggested as a significant cause of this cardiac disorder in the developed world, the limited data from Africa suggest that AIDS-related immunosuppression may increase patients' susceptibility to viral, as well as nonviral, opportunistic infections. One histological study of 16 patients suggested that nonviral opportunistic infections could have an important etiological role; Toxoplasma gondii was found in 19%, M. avium intracellulare in approximately 13% and Cryptococcus neoformans in a further 19% of participants. Myocarditis was attributed to direct HIV infection in the remainder.49 An important, unresolved question is whether people infected with HIV are highly susceptible to myocarditis from opportunistic organisms or whether a myocardial disease exists that is unique to these patients. In developed countries, numerous histological studies designed to ascertain the etiology and pathogenesis of cardiomyopathy have found lymphocytic myocarditis, with or without myocytolysis.45 Some of these studies have implicated HIV itself,43,53 and some found evidence of other cardiotropic viruses within the myocardium.42 Despite these histological findings, doubts about HIV-related myocarditis being the main cause of cardiomyopathy have persisted for a number of reasons. Many studies have used the insensitive and unspecific Dallas criteria54 to classify the histology, whilst others have not applied any accepted diagnostic criteria for myocarditis. As a result, the histological description of acute lymphocytic myocarditis might have included results that would have been interpreted differently if stringent quantitative criteria, such as the Marburg criteria,55 had been used in these studies. Because myocytes do not have the surface receptors needed for HIV entry, HIV has no recognized mechanism through which it can directly infect the heart.56 The implications, therefore, of finding HIV in cardiac tissue, by sensitive techniques (e.g. polymerase chain reaction), are not clear. Finally, patients with advanced immunosuppression often have low-grade, persistent viremia with evidence of viral particles in many tissues, regardless of tissue injury or organ dysfunction.57 The implications of finding these viral particles in patients with dilated cardiomyopathies when they are also found ubiquitously in patients with normal hearts are also not clear.
Other possible mechanisms for cardiomyopathy in people infected with HIV have been proposed. These include selenium deficiency, HAART-induced cardiotoxicity and autoimmunity. Selenium deficiency, which leads to the production of potentially cardiotoxic free radicals,58 was reported to be one of several factors associated with cardiomyopathy in a Rwandan cohort infected with HIV.36 Given that tests to measure selenium levels are not widely available in Africa, and that selenium deficiency is common in patients with AIDS,59 selenium replacement might be prudent in patients with cardiomyopathy. Nuclear reverse transcriptase inhibitors form the backbone of HAART regimens in Africa; therefore, it is important to note that the early concerns regarding their potential cardiotoxicity60 have not materialized in either developing or developed countries.
Diagnosis, prognosis and management
The symptoms, signs and results of tests, such as electrocardiography, chest radiography and echocardiography, are not altered by HIV status in patients with cardiomyopathy. Furthermore, no evidence exists that the management of heart failure should change according to HIV status. However, because the diagnosis of cardiomyopathy carries with it such a grave prognosis, and because, to date, there is no evidence that HAART reverses or improves cardiac function once cardiomyopathy is established,4 a case report of successful heart transplantation in a patient infected with HIV is encouraging.61 The reality, however, is that for the majority of patients with HIV in Africa, where the infrastructure and funds are inadequate to provide the minimum of HAART, cardiac transplantation is not a realistic treatment option.
Pulmonary hypertension
Prevalence
Pulmonary hypertension develops in a small minority of patients infected with HIV; the estimated prevalence is 0.5%, with a range of 0.1–2.0% in developed countries.62,63 Although corresponding data from Africa are unavailable, we have no reason to believe that the prevalence would be lower. Findings consistent with pulmonary hypertension, or an adaptive response to elevated pulmonary pressures, such as right ventricular hypertrophy, have been described in several echocardiography studies of people infected with HIV in Africa.34,39 Because of the relatively high incidence of pulmonary diseases, such as tuberculosis and bronchiectasis, in Africa, it has always been presumed that secondary pulmonary hypertension is much more prevalent than HIV-associated pulmonary hypertension in this population. Anecdotal experience from across the African continent indicates that patients with HIV and comorbid lung or cardiac disease develop pulmonary hypertension more often and more severely than their uninfected counterparts. Whether HIV accelerates the development of pulmonary hypertension in these patients, who might already have an underlying predisposition for pulmonary hypertension, is an intriguing and untested hypothesis.
Pathogenesis and prognosis
To date, studies from Africa have contributed little to our understanding of the pathogenesis of HIV-associated pulmonary hypertension. Anecdotal data from centers in Africa indicate little difference between the clinical manifestations of the disease in Africa and the developed world. In a study from the US, the mean length of time taken from symptom onset to diagnosis was reported to be 6 months in those with HIV, compared with more than 30 months in those without HIV.62 Despite this short time to diagnosis, the average time from diagnosis to death was reported to be less than 6 months in patients with HIV.62,64 A range of therapeutic options that provide symptomatic relief, such as prostanoids, endothelin receptor antagonists and phosphodiesterase type 5 inhibitors, are available to patients in much of the developed world; however, the cost of such care is prohibitive in most of Africa. Once the diagnosis is confirmed, the natural history is one of a rapid course to death for the majority of patients. An improved understanding of the underlying pathogenesis could lead to new therapeutic targets, and better therapeutic options than those currently available.
Coronary artery disease
In developed countries, treatment with HAART has led to an unprecedented reduction in AIDS-related mortality and a vastly improved life expectancy among people infected with HIV.65 Because patients survive to older age, ischemic heart disease has become a major contributor to morbidity and mortality.66 As a result, the prevention and management of coronary artery disease and related metabolic abnormalities have become a major focus of clinicians who look after patients with HIV.67
In Africa, although the proportion of people with access to HAART is far too small, the absolute number of people who have been on HAART since the early 1990s is close to 2 million.9 The adoption, by most of sub-Saharan Africa, of the United Nations Millennium Development goals to combat AIDS and reverse the spread of HIV by 2015 has seen the introduction of programs to increase access to HAART. These initiatives have led to a 10-fold increase in access to HAART since 2003.9 The experience gained, in patient-years, in the use of these drugs has been substantial. Despite the infrastructural impediments to the optimal delivery of health care in Africa, the beneficial effects of HAART on mortality have, in many instances, exceeded expectations.9 Interestingly, the significant increase in cardiovascular morbidity and mortality related to increased use of HAART, which has been reported in developed countries, has not occurred in Africa. Reports from several African countries have shown that morbidity and mortality amongst patients receiving HAART remains predominantly related to opportunistic infections with few, if any, reports of an increase in coronary artery disease.68,69,70,71
The reasons for this apparent discrepancy are not clear. One suggestion is that the baseline incidence of coronary disease and lipid abnormalities in the absence of HIV in sub-Saharan Africa is relatively low72,73 compared with that in developed countries. Another suggestion is that protease inhibitors, such as abacavir and didanosine, which are implicated in the increased risk of ischemic heart disease in the Data Collection on Adverse Events of Anti-HIV Drugs (DAD) publications,6,74 do not form the backbone of the main, nationally sponsored, anti-retroviral regimens in Africa, and are, therefore, infrequently used.9 Finally, perhaps the investigators and clinicians in Africa have not looked hard enough for coronary artery disease in patients on HAART.
Conclusions
The dire predictions of epidemics of acquired cardiomyopathy and coronary artery disease related to HIV have proved to be unfounded thus far;75 however, HIV-related heart disease has emerged as an important clinical entity in developed countries, as well as in Africa. In regions of high HIV seroprevalence and limited access to anti-retroviral therapy, the spectrum of HIV-related cardiac disease is dominated by pathology that is related to immunosuppression, high HIV viral load, and associated opportunistic infections and malignancies. In wealthier, opportunistic-disease-free, low seroprevalent regions, which have widespread access to anti-retroviral therapy, a growing percentage of an ageing, HIV-infected population is succumbing to chronic vascular disease. Considerable gaps in our knowledge regarding HIV-associated pericarditis, cardiomyopathy and pulmonary hypertension remain, and need to be addressed. These knowledge gaps could be filled through increased research into pathological mechanisms and pathogenesis on the one hand, and new treatment targets and options on the other. However, the virtual disappearance of these disorders as significant problems in developed countries suggests that efforts to increase the number of people with access to anti-retroviral drugs should be supported and endorsed as a main long-term objective. Finally, if the Millennium Development goals of universal access to HAART and reversal of HIV transmission can eventually be met in Africa, the day might arrive when, like our colleagues in the developed world, we can also discuss myocarditis and pericarditis as scourges of a bygone era. The current low levels of coronary artery disease associated with and without HIV and HAART in Africa also suggest that we have a unique opportunity to prevent the second wave of HAART associated cardiovascular disease experienced by our wealthier counterparts. The ongoing endeavor to elucidate the relationship between anti-retroviral therapy and risk factors for atherosclerosis and coronary disease needs to continue to avoid replacing the HIV and AIDS pandemic with that of metabolic and vascular disease.
Review criteria
A search for original articles published between 1980 and 2008 that focused on HIV and the heart was performed in MEDLINE and PubMed. The search terms used were “HIV”, “AIDS”, “Africa”, “cardiovascular disease”, “pericardial disease”, “cardiomyopathy” and “pulmonary hypertension”. We searched the reference lists of identified articles for further relevant papers. We identified and included all studies of cardiac manifestations of HIV infection in Africa. Given the lack of data from Africa, we relied on papers from the era before the introduction of HAART and our own clinical experience in some sections. Where multiple similar articles existed we selected the earliest paper to establish an original concept.
References
Autran B et al. (1983) AIDS in a Haitian woman with cardiac Kaposi's sarcoma and Whipple's disease. Lancet 1: 767–768
Ntsekhe M and Hakim J (2005) Impact of human immunodeficiency virus infection on cardiovascular disease in Africa. Circulation 112: 3602–3607
Barbarini G (2003) Incidence of the involvement of the cardiovascular system in HIV infection. AIDS 17 (Suppl): S46–S50
Pugliese A et al. (2000) Impact of highly active antiretroviral therapy in HIV-positive patients with cardiac involvement. J Infect 40: 282–284
Magula NP and Mayosi BM. (2003) Cardiac involvement in HIV-infected people living in Africa: a review. Cardiovasc J S Afr 14: 231–237
DAD Study Group et al. (2007) Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 356: 1723–1735
Mayosi BM (2007) Contemporary trends in the epidemiology and management of cardiomyopathy and pericarditis in sub-Saharan Africa. Heart 93: 1176–1183
Currie PF et al. (1995) A review of endocarditis in acquired immunodeficiency syndrome and human immunodeficiency virus infection. Eur Heart J 16 (Suppl B): 15–18
UNAIDS (online 2006) 2006 Report on the global AIDS epidemic. [http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2006/] (accessed 12 June 2008)
Reuter H et al. (2005) Epidemiology of pericardial effusions at a large academic hospital in South Africa. Epidemiol Infect 133: 393–399
Cegielski JP et al. (1990) Pericardial disease and human immunodeficiency virus in Dar es Salaam, Tanzania. Lancet 335: 209–212
Malu K et al. (1988) Pericarditis and acquired immunodeficiency syndrome [French]. Arch Mal Coeur Vaiss 81: 207–211
Taelman H et al. (1990) Pericardial effusion and HIV infection. Lancet 335, 924
Reynolds MM et al. (1992) Large pericardial effusions in the acquired immunodeficiency syndrome. Chest 102: 1746–1747
Hsia J and Ross AM. (1994) Pericardial effusion and pericardiocentesis in human immunodeficiency virus infection. Am J Cardiol 74: 94–96
Pozniak AL et al. (1994) Tuberculous pericardial effusion associated with HIV infection: a sign of disseminated disease. Tuber Lung Dis 75: 297–300
Eisenberg MJ et al. (1992) HIV-associated pericardial effusions. Chest 102: 956–958
Sagristà-Sauleda J et al. (2004) Effusive-constrictive Pericarditis. N Engl J Med 350: 469–475
Mayosi BM et al. (2006) Clinical characteristics and initial management of patients with tuberculous pericarditis in the HIV era: the Investigation of the Management of Pericarditis in Africa (IMPI Africa) registry. BMC Infect Dis 6: 2
Niakara A et al. (2001) Pericarditis in HIV infected patients: retrospective study of 40 cases in Ouagadougou, Burkina Faso [French]. Sante 11: 167–172
Mayosi BM et al. (2008) Mortality in patients treated for tuberculous pericarditis in sub-Saharan Africa. S Afr Med J 98: 36–40
Ntsekhe M et al. (2008) HIV infection is associated with a lower incidence of constriction in presumed tuberculous pericarditis: a prospective observational study. PLoS ONE 3: e2253
Mayosi BM et al. (2005) Tuberculous pericarditis. Circulation 112: 3608–3616
Syed FF and Mayosi BM (2007) A modern approach to tuberculous pericarditis. Prog Cardiovasc Dis 50: 218–236
Sagristà-Sauleda J et al. (2000) Clinical clues to the causes of large pericardial effusions. Am J Med 109: 95–101
Heidenreich PA et al. (1995) Pericardial effusion in AIDS. Incidence and survival. Circulation 92: 3229–3234
Wang SH et al. (2008) Cardiac tamponade: an unusual complication of simultaneous treatment of tuberculosis and HIV. South Med J 101: 558–560
Lawn SD et al. (2008) Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med 177: 680–685
Mayosi BM et al. (2002) Interventions for treating tuberculous pericarditis. Cochrane Database Syst Rev CD000526
Ntsekhe M et al. (2003) Adjuvant corticosteroids for tuberculous pericarditis: promising, but not proven. QJM 96: 593–599
Hakim JG et al. (2000) Double blind randomised placebo controlled trial of adjunctive prednisolone in the treatment of effusive tuberculous pericarditis in HIV seropositive patients. Heart 84: 183–188
Wiysonge CS et al. (2008) Contemporary use of adjunctive corticosteroids in tuberculous pericarditis. Int J Cardiol 124: 388–390
Dheda K et al. (2004) Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J Infect Dis 190: 1670–1676
Hakim JG et al. (1996) Myocardial dysfunction in human immunodeficiency virus infection: an echocardiographic study of 157 patients in hospital in Zimbabwe. Heart 76: 161–165
Nzuobontane D et al. (2002) Cardiac involvement in HIV infected people in Yaounde, Cameroon. Postgrad Med J 78: 678–681
Twagirumukiza M et al. (2007) Prevalence of dilated cardiomyopathy in HIV-infected African patients not receiving HAART: a multicenter, observational, prospective, cohort study in Rwanda. Curr HIV Res 5: 129–137
Longo-Mbenza B et al. (1997) The effect of HIV infection on high incidence of heart diseases in Kinshasa (Zaire). Echocardiographic study [French]. Ann Cardiol Angeiol (Paris) 46: 81–87
Felker GM et al. (2000) Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 342: 1077–1084
Niakara A et al. (2002) Cardiovascular diseases and HIV infection: study of 79 cases at the National Hospital of Ouagadougou (Burkina Faso) [French]. Bull Soc Pathol Exot 95: 23–26
Longo-Mbenza B et al. (1995) A clinical study of cardiac manifestations related to acquired immunodeficiency syndrome (AIDS) in Kinsaha [French]. Arch Mal Coeur Vaiss 88: 1437–1443
Longo-Mbenza B et al. (1998) Assessment of ventricular diastolic function in AIDS patients from Congo: a Doppler echocardiographic study. Heart 80: 184–189
Parravicini C et al. (1991) Phenotype of intramyocardial leukocytic infiltrates in acquired immunodeficiency syndrome (AIDS): a postmortem immunohistochemical study in 34 consecutive cases. Mod Pathol 4: 559–565
Grody WW et al. (1990) Infection of the heart by the human immunodeficiency virus. Am J Cardiol 66: 203–206
Flomenbaum M et al. (1989) Proliferative membranopathy and human immunodeficiency virus in AIDS hearts. J Acquir Immune Defic Syndr 2: 129–135
Baroldi G et al. (1988) Focal lymphocytic myocarditis in acquired immunodeficiency syndrome (AIDS): a correlative morphologic and clinical study in 26 consecutive fatal cases. J Am Coll Cardiol 12: 463–469
Corallo S et al. (1988) Echocardiography detects myocardial damage in AIDS: prospective study in 102 patients. Eur Heart J 9: 887–892
Barbaro G et al. (1998) Cardiac involvement in the acquired immunodeficiency syndrome: a multicenter clinical-pathological study. Gruppo Italiano per lo Studio Cardiologico dei pazienti affetti da AIDS Investigators. AIDS Res Hum Retroviruses 14: 1071–1077
Anderson DW et al. (1988) Prevalent myocarditis at necropsy in the acquired immunodeficiency syndrome. J Am Coll Cardiol 11: 792–799
Longo-Mbenza B et al. (1998) Heart involvement and HIV infection in African patients: determinants of survival. Int J Cardiol 64: 63–73
Currie PF et al. (1994) Heart muscle disease related to HIV infection: prognostic implications. BMJ 309: 1605–1607
Merx MW and Weber C (2007) Sepsis and the heart. Circulation 116: 793–802
Barbaro G et al. (2006) Takotsubo-like left ventricular dysfunction in an HIV-infected patient. Curr HIV Res 4: 239–241
Rodriguez ER et al. (1991) Cardiac myocytes and dendritic cells harbor human immunodeficiency virus in infected patients with and without cardiac dysfunction: detection by multiplex, nested, polymerase chain reaction in individually microdissected cells from right ventricular endomyocardial biopsy tissue. Am J Cardiol 68: 1511–1520
Baughman KL (2006) Diagnosis of myocarditis: death of Dallas criteria. Circulation 113: 593–595
Maisch B et al. (2000) Definition of inflammatory cardiomyopathy (myocarditis): on the way to consensus. A status report. Herz 25: 200–209
Barbaro G et al. (2001) Pathogenesis of HIV-associated cardiovascular complications. Lancet Infect Dis 1: 115–124
Carrigan DR (1997) Adenovirus infections in immunocompromised patients. Am J Med 102: 71–74
Kaul S et al. (1991) Cardiac manifestations of acquired immune deficiency syndrome: a 1991 update. Am Heart J 122: 535–544
Singhal N and Austin J (2002) A clinical review of micronutrients in HIV infection. J Int Assoc Physicians AIDS Care (Chic Ill) 1: 63–75
Domanski MJ et al. (1995) Effect of zidovudine and didanosine treatment on heart function in children infected with human immunodeficiency virus. J Pediatr 127: 137–146
Calabrese LH et al. (2003) Successful cardiac transplantation in an HIV-1-infected patient with advanced disease. N Engl J Med 348: 2323–2328
Mehta NJ et al. (2000) HIV-Related pulmonary hypertension: analytic review of 131 cases. Chest 118: 1133–1141
Sitbon O et al. (2008) Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 177: 108–113
Opravil M et al. (1997) HIV-associated primary pulmonary hypertension. A case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med 155: 990–995
Palella FJ Jr et al. (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338: 853–860
Lohse N et al. (2007) Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med 146: 87–95
Grinspoon SK et al. (2008) State of the science conference: Initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS: executive summary. Circulation 118: 198–210
Venter WD and Sanne IM (2003) The cardiovascular consequences of HIV and antiretroviral therapy. Cardiovasc J S Afr 14: 225–229
Fairall LR et al. (2008) Effectiveness of antiretroviral treatment in a South African program: a cohort study. Arch Intern Med 168: 86–93
Mermin J et al. (2008) Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. Lancet 371: 752–759
Moh R et al. (2007) Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS 21: 2483–2491
Mensah GA (2008) Ischaemic heart disease in Africa. Heart 94: 836–843
Sliwa K et al. (2008) Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet 371: 915–922
DAD Study Group et al. (2008) Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 371: 1417–1426
Maggi P et al. (2002) Premature vascular lesions in HIV-positive patients: a clockwork bomb that will explode? AIDS 16: 947–948
Acknowledgements
The authors wish to acknowledge Patrick Commerford for his unwavering support, Magdi Yacoub for the invitation to submit this review, The National Research Foundation of South Africa, Medical Research Council of South Africa, South African Heart Association, Cardiac Clinic Research Fund and Medical Education for South African Blacks (through the Don Kennedy Research Grant) for providing research grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ntsekhe, M., Mayosi, B. Cardiac manifestations of HIV infection: an African perspective. Nat Rev Cardiol 6, 120–127 (2009). https://doi.org/10.1038/ncpcardio1437
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio1437
This article is cited by
-
Venoarterial extracorporeal membrane oxygenation for cardiac support in human immunodeficiency virus-positive patients: a case report and review of a multicentre registry
Journal of Cardiothoracic Surgery (2023)
-
Pathogenesis of chronic heart failure: cardiovascular aging, risk factors, comorbidities, and disease modifiers
Heart Failure Reviews (2022)
-
The association between HIV and atherosclerotic cardiovascular disease in sub-Saharan Africa: a systematic review
BMC Public Health (2017)
-
Contributions of risk factors and medical care to cardiovascular mortality trends
Nature Reviews Cardiology (2015)
-
Prevalence of cardiovascular diseases in HIV-infected outpatients: results from a prospective, multicenter cohort study
Clinical Research in Cardiology (2013)