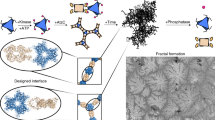
Abstract
Fractals, being “exactly the same at every scale or nearly the same at different scales” as defined by Benoit B. Mandelbrot, are complicated yet fascinating patterns that are important in aesthetics, mathematics, science and engineering. Extended molecular fractals formed by the self-assembly of small-molecule components have long been pursued but, to the best of our knowledge, not achieved. To tackle this challenge we designed and made two aromatic bromo compounds (4,4″-dibromo-1,1′:3′,1″-terphenyl and 4,4‴-dibromo-1,1′:3′,1″:4″,1‴-quaterphenyl) to serve as building blocks. The formation of synergistic halogen and hydrogen bonds between these molecules is the driving force to assemble successfully a whole series of defect-free molecular fractals, specifically Sierpiński triangles, on a Ag(111) surface below 80 K. Several critical points that govern the preparation of the molecular Sierpiński triangles were scrutinized experimentally and revealed explicitly. This new strategy may be applied to prepare and explore various planar molecular fractals at surfaces.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mandelbrot, B. B. The Fractal Geometry of Nature (W. H. Freeman, 1982).
Newkome, G. R. & Shreiner, C. Dendrimers derived from 1→3 branching motifs. Chem. Rev. 110, 6338–6442 (2010).
Sugiura, K-I., Tanaka, H., Matsumoto, T., Kawai, T. & Sakata, Y. A mandala-patterned bandanna-shaped porphyrin oligomer, C1244H1350N84Ni20O88, having a unique size and geometry. Chem. Lett. 28, 1193–1194 (1999).
Newkome, G. R. et al. Nanoassembly of a fractal polymer: a molecular ‘Sierpinski hexagonal gasket’. Science 312, 1782–1785 (2006).
Fujibayashi, K., Hariadi, R., Park, S. H., Winfree, E. & Murata, S. Toward reliable algorithmic self-assembly of DNA tiles: a fixed-width cellular automaton pattern. Nano Lett. 8, 1791–1797 (2007).
Sarkar, R. et al. One-step multicomponent self-assembly of a first-generation Sierpiński triangle: from fractal design to chemical reality. Angew. Chem. Int. Ed. 53, 12182–12185 (2014).
Wang, M. et al. Hexagon wreaths: self-assembly of discrete supramolecular fractal architectures using multitopic terpyridine ligands. J. Am. Chem. Soc. 136, 6664–6671 (2014).
Conversano, E. & Lalli, L. T. Sierpinski triangles in stone, on medieval floors in Rome. J. Appl. Math. 4, 114–122 (2011).
Devaney, R. L. Chaos and Fractals: the Mathematics behind the Computer Graphics (American Mathematical Society, 1989).
Wolfram, S. A New Kind of Science (Wolfram Media, 2001).
Alfonseca, M. & Ortega, A. Determination of fractal dimensions from equivalent L systems. IBM J. Res. Dev. 45, 797–805 (2001).
Pawin, G., Wong, K. L., Kwon, K. Y. & Bartels, L. A homomolecular porous network at a Cu(111) surface. Science 313, 961–962 (2006).
Bui, T. T. T., Dahaoui, S., Lecomte, C., Desiraju, G. R. & Espinosa, E. The nature of halogen···halogen interactions: a model derived from experimental charge-density analysis. Angew. Chem. Int. Ed. 48, 3838–3841 (2009).
Walch, H., Gutzler, R., Sirtl, T., Eder, G. & Lackinger, M. Material- and orientation-dependent reactivity for heterogeneously catalyzed carbon–bromine bond homolysis. J. Phys. Chem. C 114, 12604–12609 (2010).
Chung, K-H. et al. Polymorphic porous supramolecular networks mediated by halogen bonds on Ag(111). Chem. Commun. 47, 11492–11494 (2011).
Wang, W., Shi, X., Wang, S., Van Hove, M. A. & Lin, N. Single-molecule resolution of an organometallic intermediate in a surface-supported Ullmann coupling reaction. J. Am. Chem. Soc. 133, 13264–13267 (2011).
Fasel, R., Parschau, M. & Ernst, K-H. Amplification of chirality in two-dimensional enantiomorphous lattices. Nature 439, 449–452 (2006).
Blüm, M-C., Ćavar, E., Pivetta, M., Patthey, F. & Schneider, W-D. Conservation of chirality in a hierarchical supramolecular self-assembled structure with pentagonal symmetry. Angew. Chem. Int. Ed. 44, 5334–5337 (2005).
Hauptmann, N. et al. Surface control of alkyl chain conformations and 2D chiral amplification. J. Am. Chem. Soc. 135, 8814–8817 (2013).
Nieckarz, D. & Szabelski, P. Understanding pattern formation in 2D metal–organic coordination systems on solid surfaces. J. Phys. Chem. C 117, 11229–11241 (2013).
Nieckarz, D. & Szabelski, P. Simulation of the self-assembly of simple molecular bricks into Sierpiński triangles. Chem. Commun. 50, 6843–6845 (2014).
Röder, H., Hahn, E., Brune, H., Bucher, J-P. & Kern, K. Building one- and two-dimensional nanostructures by diffusion-controlled aggregation at surfaces. Nature 366, 141–143 (1993).
Brune, H., Romainczyk, C., Röder, H. & Kern, K. Mechanism of the transition from fractal to dendritic growth of surface aggregates. Nature 369, 469–471 (1994).
Otero, R. et al. Elementary structural motifs in a random network of cytosine adsorbed on a gold(111) surface. Science 319, 312–315 (2008).
Barth, J. V., Costantini, G. & Kern, K. Engineering atomic and molecular nanostructures at surfaces. Nature 437, 671–679 (2005).
Libbrecht, K. G. The physics of snow crystals. Rep. Prog. Phys. 68, 855–895 (2005).
Gross, L. et al. Bond-order discrimination by atomic force microscopy. Science 337, 1326–1329 (2012).
de Oteyza, D. G. et al. Direct imaging of covalent bond structure in single-molecule chemical reactions. Science 340, 1434–1437 (2013).
Chiang, C-L., Xu, C., Han, Z. & Ho, W. Real-space imaging of molecular structure and chemical bonding by single-molecule inelastic tunneling probe. Science 344, 885–888 (2014).
Blake, M. M. et al. Identifying reactive intermediates in the Ullmann coupling reaction by scanning tunneling microscopy and spectroscopy. J. Phys. Chem. A 113, 13167–13172 (2009).
Fan, Q. et al. Surface-assisted organic synthesis of hyperbenzene nanotroughs. Angew. Chem. Int. Ed. 52, 4668–4672 (2013).
Horcas, I. et al. WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 78, 013705 (2007).
Acknowledgements
This work was jointly supported by National Natural Science Foundation of China (51121091, 21133001, 21333001, 21261130090, 61321001, 913000002) and Ministry of Science and Technology (2011CB808702, 2013CB933404), China, with partial support from the Singapore National Research Foundation CREATE-SPURc. We thank X. Ma at the School of Mathematics in Peking University for his assistance and discussions on the fractal structure analysis.
Author information
Authors and Affiliations
Contributions
J.S., Y.F.W., J.M.G. and K.W. designed the experiments. J.S., M.C., J.X.D. and X.Z. performed the experiments. J.K. and G.H. synthesized the precursors. J.S., Y.F.W., X.S. and K.W. analysed the data and wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1204 kb)
Rights and permissions
About this article
Cite this article
Shang, J., Wang, Y., Chen, M. et al. Assembling molecular Sierpiński triangle fractals. Nature Chem 7, 389–393 (2015). https://doi.org/10.1038/nchem.2211
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2211
This article is cited by
-
Inner skin effects on non-Hermitian topological fractals
Communications Physics (2023)
-
Evolution of Br⋯Br contacts in enantioselective molecular recognition during chiral 2D crystallization
Nature Communications (2022)
-
Topological random fractals
Communications Physics (2022)
-
Visualization of on-surface polymerization
Science China Materials (2022)
-
Spiral fractal patterns via hierarchical assembly
Nano Research (2022)