Abstract
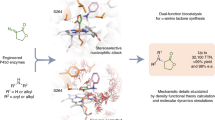
The synthesis of complex organic compounds usually relies on controlling the reactions of the functional groups. In recent years, it has become possible to carry out reactions directly on the C–H bonds, previously considered to be unreactive1,2,3. One of the major challenges is to control the site-selectivity because most organic compounds have many similar C–H bonds. The most well developed procedures so far rely on the use of substrate control, in which the substrate has one inherently more reactive C–H bond4 or contains a directing group5,6 or the reaction is conducted intramolecularly7 so that a specific C–H bond is favoured. A more versatile but more challenging approach is to use catalysts to control which site in the substrate is functionalized. p450 enzymes exhibit C–H oxidation site-selectivity, in which the enzyme scaffold causes a specific C–H bond to be functionalized by placing it close to the iron–oxo haem complex8. Several studies have aimed to emulate this enzymatic site-selectivity with designed transition-metal catalysts but it is difficult to achieve exceptionally high levels of site-selectivity9,10,11. Recently, we reported a dirhodium catalyst for the site-selective functionalization of the most accessible non-activated (that is, not next to a functional group) secondary C–H bonds by means of rhodium-carbene-induced C–H insertion12. Here we describe another dirhodium catalyst that has a very different reactivity profile. Instead of the secondary C–H bond12, the new catalyst is capable of precise site-selectivity at the most accessible tertiary C–H bonds. Using this catalyst, we modify several natural products, including steroids and a vitamin E derivative, indicating the applicability of this method of synthesis to the late-stage functionalization of complex molecules. These studies show it is possible to achieve site-selectivity at different positions within a substrate simply by selecting the appropriate catalyst. We hope that this work will inspire the design of even more sophisticated catalysts, such that catalyst-controlled C–H functionalization becomes a broadly applied strategy for the synthesis of complex molecules.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yamaguchi, J., Yamaguchi, A. D. & Itami, K. C–H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 51, 8960–9009 (2012)
Gutekunst, W. R. & Baran, P. S. C–H functionalization logic in total synthesis. Chem. Soc. Rev. 40, 1976–1991 (2011)
Davies, H. M. L. & Morton, D. Recent advances in C–H functionalization. J. Org. Chem. 81, 343–350 (2016)
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035–10074 (2016)
Zhang, F. & Spring, D. R. Arene C–H functionalisation using a removable/modifiable or a traceless directing group strategy. Chem. Soc. Rev. 43, 6906–6919 (2014)
Engle, K. M., Mei, T. S., Wasa, M. & Yu, J. Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 45, 788–802 (2012)
Doyle, M. P., Duffy, R., Ratnikov, M. & Zhou, L. Catalytic carbene insertion into C–H bonds. Chem. Rev. 110, 704–724 (2010)
Dydio, P. et al. An artificial metalloenzyme with the kinetics of native enzymes. Science 354, 102–106 (2016)
Caballero, A. et al. Catalytic functionalization of low reactive C(sp(3))-H and C(sp(2))-H bonds of alkanes and arenes by carbene transfer from diazo compounds. Dalton Trans. 44, 20295–20307 (2015)
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C–H activation for the construction of C-B bonds. Chem. Rev. 110, 890–931 (2010)
Hartwig, J. F & Larsen, M. A. Undirected, homogeneous C–H bond functionalization: challenges and opportunities. ACS Central Sci. 2, 281–292 (2016)
Liao, K., Negretti, S., Musaev, D. G., Bacsa, J. & Davies, H. M. L. Site-selective and stereoselective functionalization of unactivated C–H bonds. Nature 533, 230–234 (2016)
Davies, H. M. L., Hansen, T. & Churchill, M. R. Catalytic asymmetric C−H activation of alkanes and tetrahydrofuran. J. Am. Chem. Soc. 122, 3063–3070 (2000)
Davies, H. M. L. & Morton, D. Guiding principles for site selective and stereoselective intermolecular C–H functionalization by donor/acceptor rhodium carbenes. Chem. Soc. Rev. 40, 1857–1869 (2011)
Demonceau, A., Noels, A. F., Hubert, A. J. & Teyssie, P. Transition-metal-catalyzed reactions of diazoesters. Insertion into C–H bonds of paraffins by carbenoids. J. Chem. Soc. Chem. Commun. 14, 688–689 (1981)
Taber, D. F. & Petty, E. H. General route to highly functionalized cyclopentane derivatives by intramolecular C–H insertion. J. Org. Chem. 47, 4808–4809 (1982)
Qin, C. & Davies, H. M. L. Role of sterically demanding chiral dirhodium catalysts in site-selective C–H functionalization of activated primary C–H bonds. J. Am. Chem. Soc. 136, 9792–9796 (2014)
Guptill, D. M. & Davies, H. M. L. 2,2,2-Trichloroethyl aryldiazoacetates as robust reagents for the enantioselective C–H functionalization of methyl ethers. J. Am. Chem. Soc. 136, 17718–17721 (2014)
Davies, H. M. L., Nadeau, E., Li, Z. & Morton, D. Rhodium carbenoid induced intermolecular C–H functionalization at tertiary C–H bonds. Synlett 2009, 151–154 (2009)
Reddy, R. P. & Davies, H. M. L. Dirhodium tetracarboxylates derived from adamantylglycine as chiral catalysts for enantioselective C–H aminations. Org. Lett. 8, 5013–5016 (2006)
Yamaguchi, A. D., Chepiga, K. M., Yamaguchi, J., Itami, K. & Davies, H. M. L. Concise syntheses of dictyodendrins A and F by a sequential C–H functionalization strategy. J. Am. Chem. Soc. 137, 644–647 (2015)
Barton, D. H. R., Göktürk, A. K., Morzycki, J. W. & Motherwell, W. B. The selective oxidation of protected cholestanol derivatives using the Gif system. J. Chem. Soc. Perkin Trans. 1, 583–585 (1985)
Reese, P. B. Remote functionalization reactions in steroids. Steroids 66, 481–497 (2001)
Lindsay, V. N. G., Lin, W. & Charette, A. B. Experimental evidence for the all-up reactive conformation of chiral rhodium(II) carboxylate catalysts: enantioselective synthesis of cis-cyclopropane alpha-amino acids. J. Am. Chem. Soc. 131, 16383–16385 (2009)
Lindsay, V. N. G. & Charette, A. B. Design and synthesis of chiral heteroleptic rhodium(II) carboxylate catalysts: experimental investigation of halogen bond rigidification effects in asymmetric cyclopropanation. ACS Catal. 2, 1221–1225 (2012)
DeAngelis, A., Dmitrenko, O., Yap, G. P. A. & Fox, J. M. Chiral crown conformation of Rh(2)(S-PTTL)(4): enantioselective cyclopropanation with alpha-alkyl-alpha-diazoesters. J. Am. Chem. Soc. 131, 7230–7231 (2009)
DeAngelis, A. et al. The chiral crown conformation in paddlewheel complexes. Chem. Commun. 46, 4541–4543 (2010)
Ghanem, A., Gardiner, M. G., Williamson, R. M. & Muller, P. First X-ray structure of a N-naphthaloyl-tethered chiral dirhodium(II) complex: structural basis for tether substitution improving asymmetric control in olefin cyclopropanation. Chemistry 16, 3291–3295 (2010)
Adly, F. G., Gardiner, M. G. & Ghanem, A. Design and synthesis of novel chiral dirhodium(II) carboxylate complexes for asymmetric cyclopropanation reactions. Chemistry 22, 3447–3461 (2016)
Goto, T. et al. Highly enantioselective cyclopropenation reaction of 1-alkynes with alpha-alkyl-alpha-diazoesters catalyzed by dirhodium(II) carboxylates. Angew. Chem. Int. Ed. 50, 6803–6808 (2011)
Hansen, J. & Davies, H. M. L. High symmetry dirhodium(II) paddlewheel complexes as chiral catalysts. Coord. Chem. Rev. 252, 545–555 (2008)
Borowiak, T., Wolska, I., Brycki, B., Zielinski, A. & Kowalczyk, I. Spectroscopic properties of N-n-butyltetrachlorophthalimide and supramolecular interactions in its crystals. J. Mol. Struct. 833, 197–202 (2007)
Acknowledgements
Financial support was provided by the NSF under the CCI Center for Selective C–H Functionalization (CHE-1700982). We thank Novartis and AbbVie for supporting our research in C–H functionalization. D.G.M. gratefully acknowledges NSF MRI-R2 grant (CHE-0958205) and the use of the resources of the Cherry L. Emerson Center for Scientific Computation. The NMR and X-ray instruments used in this work were supported by the National Science Foundation (CHE 1531620 and CHE 1626172).
Author information
Authors and Affiliations
Contributions
K.L. performed the synthetic experiments. V.B. and D.G.M. conducted the computational studies. T.P. and J.B. conducted the X-ray crystallographic studies. K.L. and H.M.L.D. designed and analysed the synthetic experiments and K.L., D.G.M. and H.M.L.D. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
H.M.L.D. is a named inventor on a patent entitled “Dirhodium Catalyst Compositions and Synthetic Processes Related Thereto” (US 8,974,428, issued 10 March 2015). The other authors have no competing financial interests.
Additional information
Reviewer Information Nature thanks M. Doyle and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data – see contents page for details. (PDF 13123 kb)
Supplementary Data
This zipped file contains the cif file and CheckCIF document. (ZIP 1922 kb)
Rights and permissions
About this article
Cite this article
Liao, K., Pickel, T., Boyarskikh, V. et al. Site-selective and stereoselective functionalization of non-activated tertiary C–H bonds. Nature 551, 609–613 (2017). https://doi.org/10.1038/nature24641
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature24641
This article is cited by
-
Tunable molecular editing of indoles with fluoroalkyl carbenes
Nature Chemistry (2024)
-
Photochemical diversification of strong C(sp3)–H bonds enabled by allyl bromide and sodium fluoride
Nature Synthesis (2023)
-
Asymmetric 1,2-oxidative alkylation of conjugated dienes via aliphatic C–H bond activation
Nature Synthesis (2022)
-
Unconventional mechanism and selectivity of the Pd-catalyzed C–H bond lactonization in aromatic carboxylic acid
Nature Communications (2022)
-
Computational Study of Key Mechanistic Details for a Proposed Copper (I)-Mediated Deconstructive Fluorination of N-Protected Cyclic Amines
Topics in Catalysis (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.