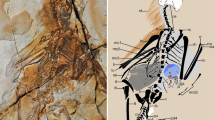
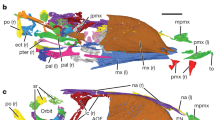
Abstract
Stem mammaliaforms are Mesozoic forerunners to mammals, and they offer critical evidence for the anatomical evolution and ecological diversification during the earliest mammalian history. Two new eleutherodonts from the Late Jurassic period have skin membranes and skeletal features that are adapted for gliding. Characteristics of their digits provide evidence of roosting behaviour, as in dermopterans and bats, and their feet have a calcaneal calcar to support the uropagatium as in bats. The new volant taxa are phylogenetically nested with arboreal eleutherodonts. Together, they show an evolutionary experimentation similar to the iterative evolutions of gliders within arboreal groups of marsupial and placental mammals. However, gliding eleutherodonts possess rigid interclavicle–clavicle structures, convergent to the avian furculum, and they retain shoulder girdle plesiomorphies of mammaliaforms and monotremes. Forelimb mobility required by gliding occurs at the acromion–clavicle and glenohumeral joints, is different from and convergent to the shoulder mobility at the pivotal clavicle–sternal joint in marsupial and placental gliders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rowe, T. B. Definition, diagnosis, and origin of Mammalia. J. Vertebr. Paleontol. 8, 241–264 (1988)
Kielan-Jaworowska, Z ., Cifelli, R. L. & Luo, Z.-X. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure (Columbia Univ. Press, New York, 2004)
Kemp, T. S. The Origin and Evolution of Mammals (Oxford Univ. Press, Oxford, 2005)
Luo, Z.-X. Transformation and diversification in early mammal evolution. Nature 450, 1011–1019 (2007)
Close, R. A., Friedman, M., Lloyd, G. T. & Benson, R. B. Evidence for a mid-Jurassic adaptive radiation in mammals. Curr. Biol. 25, 2137–2142 (2015)
Grossnickle, D. M . & Polly, P. D. Mammal disparity decreases during the Cretaceous angiosperm radiation. Proc. R. Soc. Lond. B 280, 20132110 (2013)
Ji, Q., Luo, Z. X., Yuan, C. X. & Tabrum, A. R. A swimming mammaliaform from the Middle Jurassic and ecomorphological diversification of early mammals. Science 311, 1123–1127 (2006)
Martin, T. Postcranial anatomy of Haldanodon exspectatus (Mammalia, Docodonta) from the Late Jurassic (Kimmeridgian) of Portugal and its bearing for mammalian evolution. Zool. J. Linn. Soc. 145, 219–248 (2005)
Meng, Q.-J. et al. An arboreal docodont from the Jurassic and mammaliaform ecological diversification. Science 347, 764–768 (2015)
Luo, Z.-X., Gatesy, S. M., Jenkins, F. A. Jr, Amaral, W. W. & Shubin, N. H. Mandibular and dental characteristics of Late Triassic mammaliaform Haramiyavia and their ramifications for basal mammal evolution. Proc. Natl Acad. Sci. USA 112, E7101–E7109 (2015)
Jenkins, F. A. Jr & Parrington, F. R. The postcranial skeletons of the Triassic mammals Eozostrodon, Megazostrodon and Erythrotherium. Phil. Trans. Royal Soc. B 273, 387–431 (1976)
Jenkins, F. A. Jr, Gatesy, S. M., Shubin, N. H. & Amaral, W. W. Haramiyids and Triassic mammalian evolution. Nature 385, 715–718 (1997)
Luo, Z.-X. et al. Evolutionary development in basal mammaliaforms as revealed by a docodontan. Science 347, 760–764 (2015)
Zhou, C.-F., Wu, S., Martin, T. & Luo, Z. X. A Jurassic mammaliaform and the earliest mammalian evolutionary adaptations. Nature 500, 163–167 (2013)
Zheng, X., Bi, S., Wang, X. & Meng, J. A new arboreal haramiyid shows the diversity of crown mammals in the Jurassic period. Nature 500, 199–202 (2013)
Bi, S., Wang, Y., Guan, J., Sheng, X. & Meng, J. Three new Jurassic euharamiyidan species reinforce early divergence of mammals. Nature 514, 579–584 (2014)
Dudley, R. et al. Gliding and the functional origins of flight: biomechanical novelty or necessity? Annu. Rev. Ecol. Evol. Syst. 38, 179–201 (2007)
Socha, J. J. et al. How animals glide: from trajectory to morphology. Can. J. Zool. 93, 901–924 (2015)
Jackson, S. M. Gliding Mammals of the World (CSIRO Publishing, Collingwood, Victoria, 2012)
Meng, J., Hu, Y., Wang, Y., Wang, X. & Li, C. A Mesozoic gliding mammal from northeastern China. Nature 444, 889–893 (2006)
Kermack, K. A. et al. New multituberculate-like teeth from the Middle Jurassic of England. Acta Palaeontol. Pol. 43, 581–606 (1998)
Huang, D.-Y. Yanliao Biota and Yanshan Movement. Acta Palaeontologica Sin. 54, 501–546 (2015)
Liu, Y.-Q. et al. Timing of the earliest known feathered dinosaurs and transitional pterosaurs older than the Jehol Biota. Palaeogeo. Palaeocl. Palaeoecol. 323–325, 1–12 (2012)
Yuan, C. X., Ji, Q., Meng, Q. J., Tabrum, A. R. & Luo, Z. X. Earliest evolution of multituberculate mammals revealed by a new Jurassic fossil. Science 341, 779–783 (2013)
Luo, Z.-X. et al. New evidence for mammaliaform ear evolution and feeding adaptation in a Jurassic ecosystem. Nature http://dx.doi.org/10.1038/nature23483 (2017)
Thorington, R. W. Jr & Heaney, L. R. Body proportions and gliding adaptations of flying squirrels (Petauristinae). J. Mamm. 62, 101–114 (1981)
Thorington, R. W. Jr & Santana, E. M. How to make a flying squirrel: Glaucomys anatomy in phylogenetic perspective. J. Mamm. 88, 882–896 (2007)
Johnson-Murray, J. L. The comparative myology of the gliding membranes of Acrobates, Peauroides and Petaurus contrasted with the cutaneous myology of Hemibelideus and Psudocheirus (Marsupialia, Phalangeridae) and with selected gliding Rodentia (Sciuridae and Anamoluridae). Aust. J. Zool. 35, 101–113 (1987)
Thorington, R. W. Jr & Stafford, B. J. Homologies of the carpal bones in flying squirrels (Pteromyinae): A review. Mammal Study 26, 61–68 (2001)
Kawashima, T., Murakami, K., Takayanagi, M. & Sato, F. Evolutionary transformation of the cervicobrachial plexus in the colugo (Cynocephalidae: Dermoptera) with a comparison to treeshrews (Tupaiidae: Scandentia) and strepsirrhines (Strepsirrhini: Primates). Folia Morphol. (Warsz) 71, 228–239 (2012)
Samuels, J. X. & Van Valkenburgh, B. Skeletal indicators of locomotor adaptations in living and extinct rodents. J. Morphol. 269, 1387–1411 (2008)
Chen, M. & Wilson, G. P. A multivariate approach to infer locomotor modes in Mesozoic mammals. Paleobiology 41, 280–312 (2015)
Kirk, E. C., Lemelin, P., Hamrick, M. W., Boyer, D. M. & Bloch, J. I. Intrinsic hand proportions of euarchontans and other mammals: implications for the locomotor behavior of plesiadapiforms. J. Hum. Evol. 55, 278–299 (2008)
Hammer, Ø., Harper, D. A. T. & Ryan, P. D. PAST—PAlaeontological STatistics, version 1.89. University of Oslo, Oslo 1–31 (2009)
Sues, H.-D. & Jenkins, F. A. Jr. in Amniote Paleobiology: Perspectives on the Evolution of Mammals, Birds, and Reptiles (eds M. T. Carrano et al.) 114–152 (Univ. Chicago Press, Chicago, 2006)
Luo, Z.-X. in Great Transformations: Major Events in the History of Vertebrate Life (eds K. P. Dial et al.) 167–187 (Univ. Chicago Press, Chicago, 2015)
Jenkins, F. A. Jr & Weijs, W. A. The functional anatomy of the shoulder in the Virginia opossum (Didelphis virginiana). J. Zool. 188, 379–410 (1979)
Sereno, P. C. in Amniote Paleobiology: Perspectives on the Evolution of Mammals, Birds, and Reptiles (eds M. T. Carrano et al.) 315–366 (Univ. Chicago Press, Chicago, 2006)
Szalay, F. S. Evolutionary History of the Marsupials and an Analysis of Osteological Characters (Cambridge Univ. Press, Cambridge, 1994)
Schutt, W. A. Jr & Simmons, N. B. Morphology and homology of the chiropteran calcar, with comments on the phylogenetic relationships of Archaeopteropus. J. Mamm. Evol. 5, 1–32 (1998)
Stanchak, K. E. & Santana, S. E. The calcar: a novel hind limb structure in bats. Anat. Rec. 299, 244 (2016)
Argot, C. Functional-adaptive anatomy of the forelimb in the Didelphidae, and the paleobiology of the Paleocene marsupials Mayulestes ferox and Pucadelphys andinus. J. Morphol. 247, 51–79 (2001)
Luo, Z.-X., Ji, Q., Wible, J. R. & Yuan, C. X. An Early Cretaceous tribosphenic mammal and metatherian evolution. Science 302, 1934–1940 (2003)
Simmons, N. B. & Quinn, T. H. Evolution of the digital tendon locking mechanism in bats and dermopterans: a phylogenetic perspective. J. Mamm. Evol. 2, 231–254 (1994)
Lessertisseur, J. & Saban, R. in Traité de Zoologie Tome XVI Mammiferes: Teguments et Skelettes Fascicle I (ed. Grassé, P.-P. ) 709–1078 (Masson, Paris, 1967)
Byrnes, G. & Spence, A. J. Ecological and biomechanical insights into the evolution of gliding in mammals. Integr. Comp. Biol. 51, 991–1001 (2011)
Giannini, N. P., Wible, J. R. & Simmons, N. B. On the cranial osteology of Chiroptera. I. Pteropus (Megachiroptera: Pteropodidae). Bull. Am. Mus. Nat. Hist. 295, 1–134 (2006)
Santana, S. E., Strait, S. & Dumont, E. R. The better to eat you with: functional correlates of tooth structure in bats. Funct. Ecol. 25, 839–847 (2011)
Labandeira, C. C. The pollination of Mid Mesozoic seed plants and the early history of long-proboscid insects. Ann. Mo. Bot. Gard. 97, 469–513 (2010)
Wilson, G. P. et al. Adaptive radiation of multituberculate mammals before the extinction of dinosaurs. Nature 483, 457–460 (2012)
Acknowledgements
We thank A. Shinya for fossil preparation; S. Bi, S. Gatesy, L. Heaney, H.-J. Li, Z.-J. Gao, T. Martin, B. Patterson, P. Sereno, N. Shubin, X.-T. Zheng and C.-F. Zhou for access to comparative specimens; staff of BMNH and FMNH for assistance. Research supported by funding for Q.-J.M. (Beijing Scientific Commission), Z.-X.L. (UChicago-BSD) and D.M.G. (UChicago and FMNH Fellowships). Full acknowledgments are provided in the Supplementary Information.
Author information
Authors and Affiliations
Contributions
Q.-J.M. and Z.-X.L. conceived the project; Q.-J.M., Y.-G.Z., D.L. and Q.J. acquired fossils and studied stratigraphy; all authors were involved in fossil interpretation during lab preparation; D.M.G. performed morphometric analyses; A.I.N. scanned and segmented fossils, prepared graphics; Z.-X.L. composed figures; Z.-X.L., Q.-J.M. and D.M.G. led the writing, with feedback from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Skin membranes of Maiopatagium furculiferum (holotype specimen BMNH2940).
a, Photograph under regular light. b, Photograph under UV light that enhances the fossilized soft tissue structures such as skin membranes and fur.
Extended Data Figure 2 Fossilized skin membranes of gliding eleutherodonts and comparative morphology with extant dermopteran mammals.
a, Extant dermopteran Cynocephalus: anatomical relationship of the propatagium, the manual digital webbing, and the plagiopatagium to the forelimb and hand. b, Gliding eleutherodonts: relationship of the propatagium and plagiopatagium to the forelimb and manus, based on in situ preservation of the membranes with intact forelimbs of Maiopatagium furculiferum (BMNH2940) and BMNH2942. c, d, Eleutherodont BMNH2942 with matching outlines of the propatagium, plagiopatagium and uropatagium on both slabs, BMNH2942B (c) and BMNH2942A (d). Red arrows indicate the margins of propatagium, plagiopatagium and uropatagium for BMNH2942B and the propatagium and plagiopatagium for BMNH2942A.
Extended Data Figure 3 Skull and teeth of Maiopatagium furculiferum (holotype BMNH2940).
a, b, Stereo pair photographs (a) and camera lucida drawing (b) of the dorsal view of the preserved skull. c, d, Stereo pair photos (c) and camera lucida outlines for structural identification (d) of the anterior part of the skull of Maiopatagium (BMNH2940) in ventral view as prepared from the underside of the fossil slab. e, Stereo pair images obtained by computed tomography (CT) scanning of the upper dentition of the megachiropteran fruit bat Hypsignathus in occlusal view (University of Chicago teaching collection). The bi-serial cusp rows of upper molars are functionally analogous to the upper premolars and molars of Maiopatagium. f, Occlusal view of Hypsignathus upper (purple) and lower (red) tooth rows. Note that lower molars are lingual (internal) to the upper molars (purple). g, Phytophagous phyllostomid bat Sturnira lilium (FMNH105870): upper premolars and molars with taller labial cusp row and lower lingual platform specialized for a frugivorous diet48. We consider phytophagous megachiropteran Hypsignathus and phyllostomid Sturnira to be dietary analogues to Maiopatagium and possibly to Shenshou.
Extended Data Figure 4 Shoulder girdle structures of an eleutherodont (BMNH3258).
a, Juvenile specimen that has the lower permanent premolar half-erupted to replace the deciduous premolar that is only represented by root alveoli at the ultimate premolar locus; M1 crown present but the roots not yet formed. b, CT scan images of the scapula–coracoid complex in ventral view, virtually disarticulated to show structural details. The scapular plate (red) is a composite from complementary parts preserved on the left and right scapulae. The procoracoid (green) shows the coracoid foramen and a well defined contact surface for the coracoid (blue). c, Shoulder girdle structures imaged from CT scans in ventral view. d, Shoulder girdle structures in dorsal view. Bones are coloured as follows: humerus, yellow; scapula, red; procoracoid, green; coracoid, blue; clavicles, brown; sternal series of paired manubrium, sternebrae 1–3, and a gracile xiphoid are coloured brown; the partially rendered costal ribs, brown; thoracic (dorsal) ribs, purple; vertebral column, grey. Because this is a young specimen, the interclavicle is not fully ossified. Supplementary Video 1 shows the full extent of BMNH3258 imaged by CT scans.
Extended Data Figure 5 Shoulder girdle and forelimb structures of eleutherodonts.
a, b, Maiopatagium furculiferum (holotype, BMNH2940): details of shoulder girdle and forelimbs as preserved. c, d, A new, unnamed eleutherodont BMNH2942 (see also ref. 25): preserved structures of shoulder girdle on the main slab (BMNH2942A). The interclavicle is fully ossified in BMNH2940 and BMNH2942A. The clavicles are also joined to each other and to the interclavicle in both specimens.
Extended Data Figure 6 Composite reconstruction of shoulder girdle and scapula–coracoid of eleutherodonts, in comparison to those of monotremes.
a, Reconstruction of shoulder girdle based on STL models of eleutherodont BMNH3258 (a juvenile, in ventral view); the interclavicle is not fully ossified and was reconstructed from the preserved interclavicles of BMNH2940, BMNH2942 and several other eleutherodonts with well preserved clavicule–interclavicles. b, Shoulder girdle of the monotreme Tachyglossus (adult). c, Shoulder girdle of the monotreme Ornithorhynchus (adult). d, Procoracoid, coracoid and scapula of a juvenile Ornithorhynchus. Note that the gracile coracoid, which is a juvenile feature, is similar to that of eleutherodonts. BMNH3258 is identified as a juvenile eleutherodont because it shows the lower permanent premolar in the process of erupting, and its shoulder girdle and partial forelimb elements are 80–85% the size of those on the adult specimen of Maiopatagium.
Extended Data Figure 7 Pedal structures of eleutherodonts with a bony (calcified) calcar.
a, An unnamed eleutherodont BMNH1133: right pes, showing that the calcar is distinct from, and coexists with, the os calcaris in this fossil. b, Maiopatagium (BMNH2940): right pes. Both specimens show a bony (calcified) calcar element that is articulated with the laterally bent calcaneal tuber by a contact of V-shaped trough and crest. This topographic relationship is identical to the calcar–calcaneus structural relationship of bats. Among a range of length and morphology of calcars in bats40, the eleutherodont calcar bears some resemblance to the short and stubby calcar of some bats (for example, Desmodus, among phyllostomid bats)41, although the base of the calcar is more conical and massive, distinctive from that of Desmodus41.
Extended Data Figure 8 Morphometric comparison of forelimb and manual structures of eleutherodonts and extant mammal ecomorphotypes.
a, Manual digit 3 phalangeal index. Eleutherodonts have more elongated manual phalanges than extant gliders and arborealists. b, Ternary distribution of intrinsic proportions of metacarpals, proximal phalanges, and intermediate phalanges of digit 3. Eleutherodont manual proportions are closest to those of extant mammals that are both arboreal and gliding, and are very similar to the pedal proportions of bats adapted to pedal roosting. c, Functional olecranon index, which is the ratio of olecranon length to length of the remainder of the ulna31,32. Eleutherodonts are well within the 25–75% quartiles of extant gliding mammals in having the shortest olecranon ratio, and they are below the lower 25% quartile of non-gliding arboreal mammals. The value range of this index for eleutherodonts is consistent with the interpretation that they are mostly arboreal, and some are glissant. d, Brachial index, measured as the radius length divided by humerus length31. By this index, eleutherodonts are similar to extant mammalian gliders in having high brachial index ratios, although Xianshou and extant gliders partly overlap with the 25–75% quartiles of non-gliding arboreal mammals. e, Pedal length ratio (metatarsal length/femoral length) as an index for substrate preference. Eleutherodonts are most comparable to extant gliding mammals. The index supports previous inferences of Agilodocodon, Eomaia and Sinodelphys being scansorial/arboreal, although it is less supportive of the hypothesis that Volaticotherium is a glider (see Supplementary Table 9).
Extended Data Figure 9 Morphometrics of limb skeletons of eleutherodonts and extant mammals, and inference of preferred locomotor modes.
a, Ratio of dentary length to the summed lengths of forelimb and hindlimb as an index for substrate preference, as used by Meng et al.20 to help infer that Volaticotherium is a glider. By this index, eleutherodonts are closest in values to extant gliders and arboreal mammals. However, we note that the eutriconodont Volaticotherium, the docodont Agilodocodon, and therians Eomaia and Sinodelphys, all of which had been inferred to be arboreal by qualitative morphological analyses, cannot be differentiated from extant terrestrial and semifossorial mammals by this index alone. Results suggest that Volaticotherium may not be a volant mammal, given our expanded reference dataset for this index. The efficacy of this index needs further study. b, Intermembral index is measured as the summed lengths of the humerus and radius divided by the summed lengths of the femur and tibia. Values for eleutherodonts overlap with both gliders and arborealists for this index. c, Femoral epicondyle index, which is measured as the epicondylar width divided by the femoral length (as in ref. 31). d, Crural index, which is measured as the tibial length divided by femoral length. e, PCA of six functional indices (see Supplementary Information), showing the first two components (PC1 and PC2). Purple points are eleutherodonts, and squares are non-eleutherodont Mesozoic mammals. Maiopatagium, BMNH2942 and BMNH1133 are either nested in or close to the morphospace region (purple and blue polygon) of extant mammalian gliders, suggesting that they are gliders. Volaticotherium is separated from gliders along PC2, suggesting that it may not be a glider. f, PCA showing PC1 and PC3. Maiopatagium, BMNH2942, 1133, Xianshou songae and Volaticotherium occupy the same morphospace regions as modern gliders and arboreal taxa. However, Shenshou and BMNH1137 are separated from gliders, especially along PC3, and are closely associated with non-gliding arboreal mammals.
Supplementary information
Supplementary Information
This file contains Supplementary Information parts A-J.
Video 1: Shoulder girdle and forelimb of a juvenile specimen and a comparison of the shoulder girdle of eleutherodonts and monotreme mammals.
Part 1. Shoulder girdle and forelimb of a juvenile specimen (BMNH3258). Color codes: humerus - metallic yellow; scapula – red; procoracoid – green; coracoid – blue; clavicles – brown; sternal series of paired manubrium, sternebrae 1-3 – brown, and a gracile xiphoid – brown; the partially rendered costal ribs – brown; thoracic (dorsal) ribs – purple; vertebral column – metallic grey. Part 2. Comparison of the shoulder girdle of eleutherodonts and monotreme mammals. a. Partial reconstruction of the eleutherodont shoulder girdle and sternal series, mostly based on CT scans of BMNH3258; the un-ossified interclavicle is supplemented from Maiopatagium furculiferum type specimen (BMNH2940) and other specimens. b. Adult Ornithorhynchus anatinus (the platypus). c. Juvenile Ornithorhynchus anatinus. d. Adult Tachyglossus aculeatus (the short beaked echidna). The rotation of the shoulder girdles in anatomical orientation with the sternal series is nearly horizontal.
Rights and permissions
About this article
Cite this article
Meng, QJ., Grossnickle, D., Liu, D. et al. New gliding mammaliaforms from the Jurassic. Nature 548, 291–296 (2017). https://doi.org/10.1038/nature23476
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature23476
This article is cited by
-
A large therian mammal from the Late Cretaceous of South America
Scientific Reports (2024)
-
Palaeoatmosphere facilitates a gliding transition to powered flight in the Eocene bat, Onychonycteris finneyi
Communications Biology (2024)
-
Insights into the formation and diversification of a novel chiropteran wing membrane from embryonic development
BMC Biology (2023)
-
The earliest segmental sternum in a Permian synapsid and its implications for the evolution of mammalian locomotion and ventilation
Scientific Reports (2022)
-
Sesamoids and Morphological Variation: a Hypothesis on the Origin of Rod-like Skeletal Elements in Aerial Mammals
Journal of Mammalian Evolution (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.