Abstract
Anthropogenic activities have led to large-scale mercury (Hg) pollution in the Arctic1,2,3,4,5,6. It has been suggested that sea-salt-induced chemical cycling of Hg (through ‘atmospheric mercury depletion events’, or AMDEs) and wet deposition via precipitation are sources of Hg to the Arctic in its oxidized form (Hg(ii)). However, there is little evidence for the occurrence of AMDEs outside of coastal regions, and their importance to net Hg deposition has been questioned2,7. Furthermore, wet-deposition measurements in the Arctic showed some of the lowest levels of Hg deposition via precipitation worldwide8, raising questions as to the sources of high Arctic Hg loading. Here we present a comprehensive Hg-deposition mass-balance study, and show that most of the Hg (about 70%) in the interior Arctic tundra is derived from gaseous elemental Hg (Hg(0)) deposition, with only minor contributions from the deposition of Hg(ii) via precipitation or AMDEs. We find that deposition of Hg(0)—the form ubiquitously present in the global atmosphere—occurs throughout the year, and that it is enhanced in summer through the uptake of Hg(0) by vegetation. Tundra uptake of gaseous Hg(0) leads to high soil Hg concentrations, with Hg masses greatly exceeding the levels found in temperate soils. Our concurrent Hg stable isotope measurements in the atmosphere, snowpack, vegetation and soils support our finding that Hg(0) dominates as a source to the tundra. Hg concentration and stable isotope data from an inland-to-coastal transect show high soil Hg concentrations consistently derived from Hg(0), suggesting that the Arctic tundra might be a globally important Hg sink. We suggest that the high tundra soil Hg concentrations might also explain why Arctic rivers annually transport large amounts of Hg to the Arctic Ocean9,10,11.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fitzgerald, W. F. et al. Modern and historic atmospheric mercury fluxes in northern Alaska: global sources and Arctic depletion. Environ. Sci. Technol. 39, 557–568 (2005)
Douglas, T. A. et al. The fate of mercury in Arctic terrestrial and aquatic ecosystems, a review. Environ. Chem. 9, 321–355 (2012)
Arctic Monitoring and Assessment Programme. Mercury in the Arctic (AMAP, Oslo, 2011)
Dietz, R. et al. Time trends of mercury in feathers of West Greenland birds of prey during 1851–2003. Environ. Sci. Technol. 40, 5911–5916 (2006)
Dietz, R. et al. Trend in mercury in hair of Greenlandic polar bears (Ursus maritimus) during 1892–2001. Environ. Sci. Technol. 40, 1120–1125 (2006)
Outridge, P. M. et al. A comparison of modern and pre-industrial levels of mercury in the teeth of Beluga in the Mackenzie Delta, Northwest Territories, and Walrus at Igloolik, Nunavut, Canada. Arctic 55, 123–132 (2002)
Johnson, K. P. et al. Investigation of the deposition and emission of mercury in arctic snow during an atmospheric mercury depletion event. J. Geophys. Res. 113, D17304 (2008)
National Atmospheric Deposition Program. Annual Data, all MDN sites. http://nadp.sws.uiuc.edu/data/mdn/annual.aspx (accessed Oct 3, 2016)
Schuster, P. F. et al. Mercury export from the Yukon river basin and potential response to a changing climate. Environ. Sci. Technol. 45, 9262–9267 (2011)
Fisher, J. A. et al. Riverine source of Arctic Ocean mercury inferred from atmospheric observations. Nat. Geosci. 5, 499–504 (2012)
Dastoor, A. P. & Durnford, D. A. Arctic Ocean: is it a sink or a source of atmospheric mercury? Environ. Sci. Technol. 48, 1707–1717 (2014)
Krabbenhoft, D. & Sunderland, E. M. Global change and mercury. Science 341, 1457–1458 (2013)
Polyakov, I. V. et al. Observationally based assessment of polar amplification of global warming. Geophys. Res. Lett. 29, 1878 (2002)
Arctic Climate Impact Assessment. Impacts of a Warming Arctic: Arctic Climate Impact Assessment Overview Report (Cambridge Univ. Press, 2004)
Selin, N. E. Global change and mercury cycling: challenges for implementing a global treaty. Environ. Toxicol. Chem. 33, 1202–1210 (2014)
Smith-Downey, N. V., Sunderland, E. M. & Jacob, D. J. Anthropogenic impacts on global storage and emissions of mercury from terrestrial soils: insights from a new global model. J. Geophys. Res. Biogeosci. 115, G03008 (2010)
Steffen, A. et al. A synthesis of atmospheric mercury depletion event chemistry in the atmosphere and snow. Atmos. Chem. Phys. 8, 1445–1482 (2008)
Agnan, Y. et al. New constraints on terrestrial surface-atmosphere fuxes of gaseous elemental mercury using a global database. Environ. Sci. Technol. 50, 507–524 (2016)
Demers, J. D., Blum, J. D. & Zak, D. R. Mercury isotopes in a forested ecosystem: implications for air-surface exchange dynamics and the global mercury cycle. Glob. Biogeochem. Cycles 27, 222–238 (2013)
Jiskra, M. et al. Mercury deposition and re-emission pathways in boreal forest soils investigated with Hg isotope signatures. Environ. Sci. Technol. 49, 7188–7196 (2015)
Enrico, M. et al. Atmospheric mercury transfer to peat bogs dominated by gaseous elemental mercury dry deposition. Environ. Sci. Technol. 50, 2405–2412 (2016)
Zheng, W., Obrist, D., Weis, D. & Bergquist, B. A. Mercury isotope compositions across North American forests. Glob. Biogeochem. Cycles 30, 1475–1492 (2016)
Sherman, L. S. et al. Mass-independent fractionation of mercury isotopes in Arctic snow driven by sunlight. Nat. Geosci. 3, 173–177 (2010)
Biswas, A. et al. Natural mercury isotope variation in coal deposits and organic soils. Environ. Sci. Technol. 42, 8303–8309 (2008)
Obrist, D., Pokharel, A. K. & Moore, C. Vertical profile measurements of soil air suggest immobilization of gaseous elemental mercury in mineral soil. Environ. Sci. Technol. 48, 2242–2252 (2014)
Obrist, D. et al. Mercury distribution across 14 U.S. Forests. Part I: spatial patterns of concentrations in biomass, litter, and soils. Environ. Sci. Technol. 45, 3974–3981 (2011)
Smith, D. B . et al. Geochemical and mineralogical data for soils of the coterminous United States. US Geological Survey Data Series 801, https://pubs.usgs.gov/ds/801/ (2013)
Amos, H. M. et al. Observational and modeling constraints on global anthropogenic enrichment of mercury. Environ. Sci. Technol. 49, 4036–4047 (2015)
Hararuk, O., Obrist, D. & Luo, Y. Modelling the sensitivity of soil mercury storage to climate-induced changes in soil carbon pools. Biogeosciences 10, 2393–2407 (2013)
Sprovieri, F. et al. Atmospheric mercury concentrations observed at ground-based monitoring sites globally distributed in the framework of the GMOS network. Atmos. Chem. Phys. 16, 11915–11935 (2016)
Fritsche, J. et al. Elemental mercury fluxes over a sub-alpine grassland determined with two micrometeorological methods. Atmos. Environ. 42, 2922–2933 (2008)
Castro, M. & Moore, C. Importance of gaseous elemental mercury fluxes in western Maryland. Atmosphere 7, 110 (2016)
Douglas, T. A. et al. Influence of snow and ice crystal formation and accumulation on mercury deposition to the Arctic. Environ. Sci. Technol. 42, 1542–1551 (2008)
Cole, A. S. et al. Ten-year trends of atmospheric mercury in the high Arctic compared to Canadian sub-Arctic and mid-latitude sites. Atmos. Chem. Phys 13, 1535 (2013)
Seok, B. et al. An automated system for continuous measurements of trace gas fluxes through snow: an evaluation of the gas diffusion method at a subalpine forest site, Niwot Ridge, Colorado. Biogeochemistry 95, 95–113 (2009)
Faïn, X. et al. Mercury dynamics in the Rocky Mountain, Colorado, snowpack. Biogeosciences 10, 3793–3807 (2013)
Moore, C. W., Obrist, D. & Luria, M. Atmospheric mercury depletion events at the Dead Sea: spatial and temporal aspects. Atmos. Environ. 69, 231–239 (2013)
Edwards, G. C. et al. Development and evaluation of a sampling system to determine gaseous mercury fluxes using an aerodynamic micrometeorological gradient method. J. Geophys. Res. 110, D10306 (2005)
Lyman, S. N. & Jaffe, D. A. Formation and fate of oxidized mercury in the upper troposphere and lower stratosphere. Nat. Geosci. 5, 114–117 (2012)
Zhang, L., Wright, L. P. & Blanchard, P. A review of current knowledge concerning dry deposition of atmospheric mercury. Atmos. Environ. 43, 5853–5864 (2009)
Shaver, G. R. & Chapin, F. S. Production: biomass relationships and element cycling in contrasting Arctic vegetation types. Ecol. Monogr. 61, 1–31 (1991)
Chapin, F. S., Shaver, G. R., Giblin, A. E., Nadelhoffer, K. J. & Laundre, J. A. Responses of Arctic tundra to experimental and observed changes in climate. Ecology 76, 694–711 (1995)
Fu, X., Heimburger, L.-E. & Sonke, J. E. Collection of atmospheric gaseous mercury for stable isotope analysis using iodine- and chlorine-impregnated activated carbon traps. J. Anal. At. Spectrom. 29, 841–852 (2014)
Bergquist, B. A. & Blum, J. D. Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science 318, 417–420 (2007)
Acknowledgements
We thank Toolik Field Station and Polar Field Services staff for their support in setting up the field site and maintaining its operation for two years, with special thanks to J. Timm. We thank O. Dillon and C. Pearson for support with laboratory analyses; A. Steffen and S. Brooks for providing additional instrumentation; J. Chmeleff for support with inductively coupled plasma mass spectrometry; and R. Kreidberg and J. Arnone for editorial and technical assistance in manuscript preparation. The project was funded primarily by a US National Science Foundation (NSF) award (PLR 1304305), with additional support provided by further NSF (CHN 1313755) and US Department of Energy (DE-SC0014275) awards. The Hg isotope work was funded by H2020 Marie Sklodowska-Curie grant agreement no. 657195 to M.J., and European Research Council grant ERC-2010-StG_20091028 and CNRS-INSU-CAF funding (PARCS project) to J.E.S.
Author information
Authors and Affiliations
Contributions
D.O. and D.H. initiated and designed this project, and M.J., J.E.S. and D.O. designed and developed the isotope component. All authors were involved in all field sampling and/or laboratory analyses. Y.A. led data analysis of flux data, and M.J. led stable isotope sampling and analysis with support from J.E.S. D.O. led manuscript writing with major support from M.J., Y.A., J.E.S. and C.W.M.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks J. Blum and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
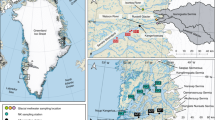
Extended Data Figure 1 Research site and measurement systems used in this study.
a, The research site was characterized as Tussock tundra, and is located at Toolik Field Station in northern Alaska, USA (68° 38′ N, 149° 36′ W). b, A temperature-controlled research laboratory was set up to house analytical sensors, control systems, and data acquisition. Heated conduits were installed to run electrical lines and trace-gas lines to the tundra and to protect lines from bite damage and freezing. c, Continuous gaseous Hg(0) flux measurements were conducted using an aerodynamic method based on gradient measurements at heights of 61 cm and 363 cm above ground. d, A snow tower, consisting of five trace-gas inlets, was established over the tundra before the onset of snowfall. Wintertime snowfall buried this tower and allowed sampling of snow air from the respective air inlets without disturbing the snowpack. e, A soil trace-gas system, consisting of six trace-gas wells at various depths and locations, was deployed in tundra soils to allow three daily extractions of soil pore gas and analysis for Hg(0) gas and auxiliary trace gases. Panel e shows an example of the wells used in year one; these were replaced with a different system in year two but detected similar gas magnitudes and seasonal patterns.
Extended Data Figure 2 Measured atmospheric Hg(ii) concentrations from February to September 2016, and estimated Hg(ii) dry deposition.
Hg(ii) concentrations were measured by using differential measurements of gaseous Hg(0) and total atmospheric Hg, which passed through a glass-tube inlet directly into a pyrolyzer oven set at 650 °C. Cumulative Hg(ii) deposition was calculated on the basis of reported deposition velocities for Hg(ii) forms of 1.5 cm s−1.
Extended Data Figure 3 Example of a springtime AMDE at Toolik Field Station, from 1–3 April 2016.
This atmospheric Hg measurement sequence shows corresponding concentration depletions of Hg(0) (in blue) and O3 (in red), along with the formation of atmospheric Hg(ii) (in green). Hg(0) concentrations reached values below the detection limit when Hg(ii) concentrations increased to 0.5 ng m−3. At the same time, O3 mixing ratios dropped down from 45 ppb to less than 10 ppb.
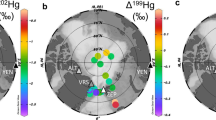
Extended Data Figure 4 Mass-independent anomalies of even-mass-number Hg isotopes (Δ200Hg) and mass-dependent Hg isotopic signatures (δ202Hg) for different Hg sources and ecosystem sinks.
Symbols for sources include: circles for atmospheric Hg(0); filled blue triangles for Hg(ii) in snow deposited before January/February 2016; open blue triangles for Hg measured in surface snow during periods of AMDEs (March/April 2016); and grey inverted triangles for geogenic Hg in bedrock samples. Symbols for tundra components include: filled diamonds for bulk vegetation; filled squares for organic (O horizon) soils; and open squares with a cross for mineral soil A horizons (which have more than 10% organic matter) or without cross for B horizons (which have less than 10% organic matter). Measurement uncertainties, calculated as 2 s.d. of replicate standards, are shown on the lower right.
Extended Data Figure 5 Mass-independent Hg isotopic anomalies (Δ199Hg and Δ201Hg) for different Hg sources and ecosystem sinks.
Symbols for sources include: circles for atmospheric Hg(0); filled blue triangles for Hg(ii) in snow deposited before January/February 2016; open blue triangles for Hg deposited during AMDEs (March/April 2016); and grey inverted triangles for geogenic Hg in bedrock samples. Symbols for tundra components include: filled diamonds for bulk vegetation; filled squares for organic (O horizon) soils; and open squares with a cross for mineral soil A horizons (more than 10% organic matter) or without a cross for B horizons (less than 10% organic matter). Measurement uncertainties, calculated as 2 s.d. of replicate standards, are shown on the lower right. All data are within analytical uncertainty, with the straight line representing the 1:1 Δ199Hg to Δ201Hg slope, thought to be representative of photochemically induced mass-independent fractionation by magnetic isotope effects44.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Tables 1-6 and Supplementary References. (PDF 494 kb)
Rights and permissions
About this article
Cite this article
Obrist, D., Agnan, Y., Jiskra, M. et al. Tundra uptake of atmospheric elemental mercury drives Arctic mercury pollution. Nature 547, 201–204 (2017). https://doi.org/10.1038/nature22997
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22997
This article is cited by
-
Retention properties and mechanism of agricultural waste maize whisker on atmospheric mercury
Bioresources and Bioprocessing (2023)
-
Comparing ecosystem gaseous elemental mercury fluxes over a deciduous and coniferous forest
Nature Communications (2023)
-
A 1500-year record of mercury isotopes in seal feces documents sea ice changes in the Antarctic
Communications Earth & Environment (2023)
-
Mercury deposition and redox transformation processes in peatland constrained by mercury stable isotopes
Nature Communications (2023)
-
Interdecadal variations of the mercury content in Scomber colias in Canary Islands
Environmental Science and Pollution Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.