Abstract
A highly protective malaria vaccine would greatly facilitate the prevention and elimination of malaria and containment of drug-resistant parasites1. A high level (more than 90%) of protection against malaria in humans has previously been achieved only by immunization with radiation-attenuated Plasmodium falciparum (Pf) sporozoites (PfSPZ) inoculated by mosquitoes2,3,4; by intravenous injection of aseptic, purified, radiation-attenuated, cryopreserved PfSPZ (‘PfSPZ Vaccine’)5,6; or by infectious PfSPZ inoculated by mosquitoes to volunteers taking chloroquine7,8,9,10 or mefloquine11 (chemoprophylaxis with sporozoites). We assessed immunization by direct venous inoculation of aseptic, purified, cryopreserved, non-irradiated PfSPZ (‘PfSPZ Challenge’12,13) to malaria-naive, healthy adult volunteers taking chloroquine for antimalarial chemoprophylaxis (vaccine approach denoted as PfSPZ-CVac)14. Three doses of 5.12 × 104 PfSPZ of PfSPZ Challenge12,13 at 28-day intervals were well tolerated and safe, and prevented infection in 9 out of 9 (100%) volunteers who underwent controlled human malaria infection ten weeks after the last dose (group III). Protective efficacy was dependent on dose and regimen. Immunization with 3.2 × 103 (group I) or 1.28 × 104 (group II) PfSPZ protected 3 out of 9 (33%) or 6 out of 9 (67%) volunteers, respectively. Three doses of 5.12 × 104 PfSPZ at five-day intervals protected 5 out of 8 (63%) volunteers. The frequency of Pf-specific polyfunctional CD4 memory T cells was associated with protection. On a 7,455 peptide Pf proteome array, immune sera from at least 5 out of 9 group III vaccinees recognized each of 22 proteins. PfSPZ-CVac is a highly efficacious vaccine candidate; when we are able to optimize the immunization regimen (dose, interval between doses, and drug partner), this vaccine could be used for combination mass drug administration and a mass vaccination program approach to eliminate malaria from geographically defined areas.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
malERA Consultative Group on Vaccines. A research agenda for malaria eradication: vaccines. PLoS Med. 8, e1000398 (2011)
Clyde, D. F., Most, H., McCarthy, V. C. & Vanderberg, J. P. Immunization of man against sporozite-induced falciparum malaria. Am. J. Med. Sci. 266, 169–177 (1973)
Rieckmann, K. H., Carson, P. E., Beaudoin, R. L., Cassells, J. S. & Sell, K. W. Letter: Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 68, 258–259 (1974)
Hoffman, S. L. et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 185, 1155–1164 (2002)
Seder, R. A. et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341, 1359–1365 (2013)
Ishizuka, A. S. et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat. Med. 22, 614–623 (2016)
Bijker, E. M. et al. Cytotoxic markers associate with protection against malaria in human volunteers immunized with Plasmodium falciparum sporozoites. J. Infect. Dis. 210, 1605–1615 (2014)
Bijker, E. M. et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc. Natl Acad. Sci. USA 110, 7862–7867 (2013)
Roestenberg, M. et al. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 361, 468–477 (2009)
Roestenberg, M. et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377, 1770–1776 (2011)
Bijker, E. M. et al. Sporozoite immunization of human volunteers under mefloquine prophylaxis is safe, immunogenic and protective: a double-blind randomized controlled clinical trial. PLoS One 9, e112910 (2014)
Gómez-Pérez, G. P. et al. Controlled human malaria infection by intramuscular and direct venous inoculation of cryopreserved Plasmodium falciparum sporozoites in malaria-naive volunteers: effect of injection volume and dose on infectivity rates. Malar. J. 14, 306 (2015)
Mordmüller, B. et al. Direct venous inoculation of Plasmodium falciparum sporozoites for controlled human malaria infection: a dose-finding trial in two centres. Malar. J. 14, 117 (2015)
Bastiaens, G. J. et al. Safety, immunogenicity, and protective efficacy of intradermal immunization with aseptic, purified, cryopreserved Plasmodium falciparum sporozoites in volunteers under chloroquine prophylaxis: a randomized controlled trial. Am. J. Trop. Med. Hyg. 94, 663–673 (2016)
Beaudoin, R. L., Strome, C. P. A., Mitchell, F. & Tubergen, T. A. Plasmodium berghei: immunization of mice against the ANKA strain using the unaltered sporozoite as an antigen. Exp. Parasitol. 42, 1–5 (1977)
Guerin-Marchand, C. et al. A liver-stage-specific antigen of Plasmodium falciparum characterized by gene cloning. Nature 329, 164–167 (1987)
Orito, Y. et al. Liver-specific protein 2: a Plasmodium protein exported to the hepatocyte cytoplasm and required for merozoite formation. Mol. Microbiol. 87, 66–79 (2013)
Tarun, A. S. et al. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc. Natl Acad. Sci. USA 105, 305–310 (2008)
Richie, T. L. et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine 33, 7452–7461 (2015)
Epstein, J. E. et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science 334, 475–480 (2011)
Suhrbier, A., Winger, L. A., Castellano, E. & Sinden, R. E. Survival and antigenic profile of irradiated malarial sporozoites in infected liver cells. Infect. Immun. 58, 2834–2839 (1990)
Behet, M. C. et al. Sporozoite immunization of human volunteers under chemoprophylaxis induces functional antibodies against pre-erythrocytic stages of Plasmodium falciparum. Malar. J. 13, 136 (2014)
Schofield, L. et al. γ Interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330, 664–666 (1987)
Weiss, W. R., Sedegah, M., Beaudoin, R. L., Miller, L. H. & Good, M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl Acad. Sci. USA 85, 573–576 (1988)
Doolan, D. L. & Hoffman, S. L. The complexity of protective immunity against liver-stage malaria. J. Immunol. 165, 1453–1462 (2000)
Belnoue, E. et al. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 172, 2487–2495 (2004)
Weiss, W. R. & Jiang, C. G. Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLoS One 7, e31247 (2012)
Fernandez-Ruiz, D. et al. Liver-resident memory CD8+ T cells form a front-line defense against malaria liver-stage infection. Immunity 45, 889–902 (2016)
Schats, R. et al. Heterologous protection against malaria after immunization with Plasmodium falciparum sporozoites. PLoS One 10, e0124243 (2015)
Egan, J. E. et al. Humoral immune responses in volunteers immunized with irradiated Plasmodium falciparum sporozoites. Am. J. Trop. Med. Hyg. 49, 166–173 (1993)
Epstein, J. E. et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight 2, e89154 (2017)
Lyke, K. et al. PfSPZ Vaccine induces strain-transcending T cells and durable protection against heterologous malaria challenge. Proc. Natl Acad. Sci. USA (in the press)
Sissoko, M. S. et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed Malian adults: a randomised, double-blind trial. Lancet Infect Dis. (in the press)
Roestenberg, M. et al. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am. J. Trop. Med. Hyg. 88, 5–13 (2013)
Sahu, T. et al. Chloroquine neither eliminates liver stage parasites nor delays their development in a murine chemoprophylaxis vaccination model. Front. Microbiol. 6, 283 (2015)
Fairley, N. H. Chemotherapeutic suppression and prophylaxis in malaria. Trans. R. Soc. Trop. Med. Hyg. 38, 311–365 (1945)
Planche, T. et al. Comparison of methods for the rapid laboratory assessment of children with malaria. Am. J. Trop. Med. Hyg. 65, 599–602 (2001)
Kamau, E., Alemayehu, S., Feghali, K. C., Saunders, D. & Ockenhouse, C. F. Multiplex qPCR for detection and absolute quantification of malaria. PLoS One 8, e71539 (2013)
Bustin, S. A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622 (2009)
R Core Team. R: A Language and Environment for Statistical Computing. (2015)
Roederer, M., Nozzi, J. L. & Nason, M. C. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 79, 167–174 (2011)
Sattabongkot, J. et al. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am. J. Trop. Med. Hyg. 74, 708–715 (2006)
Felgner, P. L. et al. Pre-erythrocytic antibody profiles induced by controlled human malaria infections in healthy volunteers under chloroquine prophylaxis. Sci. Rep. 3, 3549 (2013)
Davies, D. H. et al. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc. Natl Acad. Sci. USA 102, 547–552 (2005)
Lamoreaux, L., Roederer, M. & Koup, R. Intracellular cytokine optimization and standard operating procedure. Nat. Protocols 1, 1507–1516 (2006)
Acknowledgements
The authors thank the vaccine trial participants for their contribution and commitment to vaccine research. We thank F. Adomat, S. Adukpo, M. Aldejohann, S. Bolte, S. Borrmann, A. Bouyoukou Hounkpatin, S. Brückner, E. Bruske, J. Fernandes, P. Granados Bayón, J. Hass, S. Jeyaraj, J. Keim, A. Knoblich, R. Köllner, A. Kreidenweiss, D. N. Ndungu, R. Ritter, J. A. Selvaraj, Z. Sulyok, S. Theil, N. Theurer, and I. Westermann for support in conducting the trial, and P. Darrah and M. Roederer for assistance with the interpretation of the T-cell data. We thank the Sanaria and Protein Potential teams for manufacture and shipping of investigational products, PfSPZ Challenge and diluents, regulatory, quality, and clinical site activities, and legal and administrative support, including especially D. Cheney (née Padilla), Y. Abebe, E. Saverino, Y. Wu, E. Fomumbod, A. Awe, M. King, M. Orozco, A. Patil, Y. Wen, K. Nelson, J. Overby, S. Matheny, V. Pitch, B. Jiang, L. Gao, R. Xu, T. T. Wai, S. Monsheimer, P. De La Vega, M. Laskowski, H. Huang, M. Marquette, J. Jackson, F. Beams, R. Douglas, R. C. Thompson, D. Dolberg and A. Hoffman. We thank J. Inglese and P. Dranchak of the National Center for Advancing Translational Sciences (NCATS), NIH for support with the automated immunofluorescence assay and inhibition of sporozoite invasion assays. We appreciate the expert reviews of the Safety Monitoring Committee (W. Chen, P. Coyne and P. Zanger). The clinical trial was funded by the German Federal Ministry of Education and Research (BMBF) through the German Center for Infection Research (DZIF). Manufacture of investigational product by Sanaria was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under SBIR award numbers 5R44AI058375 and 5R44AI055229. T cell studies were supported by the intramural research program of the VRC, NIAID, NIH. Proteome microarray studies were supported by NIAID SBIR grant 5R44AI066791 and funding from the Bill & Melinda Gates Foundation.
Author information
Authors and Affiliations
Contributions
B.M. designed the study, analysed the data, contributed to data collection and wrote the manuscript; B.K.L.S., E.R.J., A.J.R., A.G.E., T.L., R.E.S. and M.L manufactured the investigational products; A.M., R.S., T.M., A.G. and P.F.B. assured quality and regulatory compliance; C.L.C. and J.B.M. performed PfSPZ formulations; G.S., M.G., M.S. and H.L. collected data; A.L., M.E., J.I., T.G. and J.H. performed laboratory analyses; S.C., N.K, M.L. and A.J.R. performed and analysed all ELISA, IFA, and inhibition of sporozoite invasion studies; J.J.C. and P.L.F. performed and analysed protein array data; A.S.I. and R.A.S. performed and analysed cytometry experiments; T.L.R. oversaw the clinical trial; A.J.R., T.L.R., P.F.B., B.K.L.S. and S.L.H. analysed data; S.L.H. and P.G.K supervised the project, interpreted data, and wrote the manuscript. S.L.H. was the clinical trial sponsor representative and B.M. the principal investigator of the trial. S.L.H. and P.G.K. contributed equally to the work. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.C., A.J.R., E.R.J., P.F.B., N.K, A.M., T.M., A.G., A.G.E., T.L., R.E.S, M.L., T.L.R., B.K.L.S. and S.L.H. are salaried employees of Sanaria Inc., the developer and owner of PfSPZ Challenge and the sponsor of the clinical trial. In addition, S.L.H. and B.K.L.S. have a financial interest in Sanaria Inc. J.J.C. is an Antigen Discovery Employee. P.L.F. owns stock and is a board member at Antigen Discovery, Inc. All other authors declare no conflicts of interest.
Additional information
Reviewer Information Nature thanks L. Rénia and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Distribution of adverse events.
a, b, The number of adverse events (AEs) regardless of attribution to investigational product. Each bar represents one volunteer sorted on the number of adverse events from the time of first injection with normal saline (controls) or PfSPZ-CVac until the time of CHMI, approximately 17 weeks later (a) and adverse events in the same volunteers from initiation of CHMI until the end of follow-up (b). Mild (grade 1) adverse events are depicted in grey, moderate (grade 2) in yellow and severe (grade 3) in blue. Non-protected volunteers are marked with an ‘M’ on the x axis.
Extended Data Figure 3 CD4 T-cell cytokine polyfunctionality.
PBMCs from subjects were drawn 14 days after third immunization (post-imm) or 1 day before CHMI (pre-CHMI), stimulated with PfSPZ, PfRBC, or stimulation controls, and stained for intracellular cytokine expression. a, b, The pie charts show the proportion of memory CD4 T cells expressing any combination of IFN-γ, IL-2, or TNF-α for each dose group after stimulation with PfSPZ (a) or PfRBC (b). Responses are background subtracted from control antigen stimulations 1% HSA or uninfected erythrocytes. c, d, The magnitude of the memory CD4 T-cell response for each combination of cytokines is shown in c and d. There is a trend towards higher polyfunctionality as dose increases. e, f, The median fluorescence intensity (MFI) for IFN-γ is shown for the different combination of IFN-γ+ cells following PfSPZ or PfRBC stimulation. Cells that simultaneously produce IFN-γ, IL-2, and TNF-α have the highest IFN-γ MFI.
Extended Data Figure 4 T-cell immunogenicity.
a, b, Memory CD4 T cells producing IL-4 (a) or IL-10 (b) after PfRBC stimulation. Memory γδ T cells producing IFN-γ, IL-2, or TNF-α following PfSPZ (c) or PfRBC stimulation (d). For a–d, results are the percentage of cytokine-producing cells after incubation with PfSPZ minus the percentage of cells after incubation with vaccine diluent (medium with 1% HSA) as control or percentage of cytokine-producing cells after incubation with asexual Pf-infected red blood cells (PfRBC) minus uninfected RBCs as control. e, f, Total memory γδ T cells assessed before immunization (pre-imm) and 14 days after third immunization (post-imm) for the percentage of cells expressing CD38. The absolute frequencies are shown in e and the change from pre-vaccination to post-vaccination is shown in f. For a–d, within a dose group, the difference from pre-vaccine was assessed by two-way ANOVA with Bonferroni correction. Data are median ± interquartile range. For e, f, difference from pre-vaccine was assessed by Wilcoxon signed rank test. P values were corrected for multiple comparisons by the Bonferroni method. *P < 0.05, *P < 0.01. Data are median ± interquartile range. Pre-imm, 3 days before first immunization; post-imm, 14 days after third immunization; pre-CHMI, 1 day before CHMI.
Extended Data Figure 5 Sub-family analysis of γδ T cells.
a–f, The frequency of the circulating γδ T-cell subsets as a percentage of total lymphocytes was assessed in unstimulated PBMCs before the first immunization (pre-imm), 2 weeks after final immunization (post-imm), and the day before CHMI (pre-CHMI). Fold change compared to pre-imm is shown for total memory γδ T cells (a), Vγ9+Vδ2+ (b), Vγ9+Vδ1+ (c), Vγ9−Vδ1+ (d), Vγ9+Vδ1−Vδ2− (e), and Vγ9−Vδ1−Vδ2− (f) subfamilies. The frequency of Vγ9−Vδ2+ subset is low to undetectable. Within a dose group, the difference from pre-imm was assessed by two-way ANOVA with Bonferroni correction. *P < 0.05, **P < 0.01. Data are geometric mean ± 95% CI.
Extended Data Figure 6 Anti-plasmodial antibody responses in vaccinated volunteers who were immunized with three doses of 5.12 × 104 PfSPZ at 28-day, 14-day, or 5-day intervals.
Antibodies to PfCSP by ELISA were assessed in sera taken before any immunizations (pre-immunization), two weeks following last immunization (post-immunization) and 10 weeks after last immunization, which was one day before CHMI (pre-CHMI). PfCSP ELISA results are reported as net OD 1.0; the reciprocal serum dilution at which the optical density was 1.0 in post-immunization or pre-CHMI sera minus the OD 1.0 in pre-immunization sera. All values met criteria for positivity. Protected volunteers are represented by yellow circles and unprotected volunteers by grey circles.
Extended Data Figure 7 Development of PfSPZ Vaccine and PfSPZ-CVac in hepatocytes.
Radiation-attenuated PfSPZ in PfSPZ Vaccine invade hepatocytes and partially develop, but do not replicate. They are metabolically active and non-replicating. Infectious PfSPZ in PfSPZ-CVac invade hepatocytes and fully develop. A single PfSPZ replicates exponentially producing more than 104 merozoites. These merozoites are released into the circulation, and each merozoite can invade a different erythrocyte. Chloroquine prevents complete parasite development within erythrocytes, thereby preventing the development of merozoites that can invade new erythrocytes.
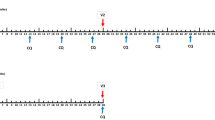
Extended Data Figure 8 Transient parasitaemia following vaccination at 5-day intervals.
Parasitaemia measured by qPCR over 22 days. The subjects who were protected and not protected against CHMI are in yellow and grey, respectively. The times of PfSPZ inoculations are shown as vertical red lines and the time of last CQ administration as a vertical blue line. CQ was given as 10 mg kg−1 (maximum 620 mg) loading dose on day 0 followed by 5 mg kg−1 (maximum 310 mg) chloroquine base on days 5, 10, and 15.
Supplementary information
Supplementary Information
This file contains Supplementary Figure 1, Supplementary Tables 1-5 and 7-9. (PDF 804 kb)
Supplementary Table 6
Supplementary Table 6 shows logistic regression of peak antibody levels (2 weeks after final immunization) and baseline antibody levels on probability of sterile protection against CHMI, adjusted by dose of PfSPZ-CVac. (PDF 1802 kb)
Rights and permissions
About this article
Cite this article
Mordmüller, B., Surat, G., Lagler, H. et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 542, 445–449 (2017). https://doi.org/10.1038/nature21060
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature21060
This article is cited by
-
Immunological factors linked to geographical variation in vaccine responses
Nature Reviews Immunology (2024)
-
Accelerated prime-and-trap vaccine regimen in mice using repRNA-based CSP malaria vaccine
npj Vaccines (2024)
-
Malaria blood stage infection suppresses liver stage infection via host-induced interferons but not hepcidin
Nature Communications (2024)
-
Ultra-low volume intradermal administration of radiation-attenuated sporozoites with the glycolipid adjuvant 7DW8-5 completely protects mice against malaria
Scientific Reports (2024)
-
A replication competent Plasmodium falciparum parasite completely attenuated by dual gene deletion
EMBO Molecular Medicine (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.