Abstract
Induced pluripotent stem cells (iPSCs) constitute a potential source of autologous patient-specific cardiomyocytes for cardiac repair, providing a major benefit over other sources of cells in terms of immune rejection. However, autologous transplantation has substantial challenges related to manufacturing and regulation. Although major histocompatibility complex (MHC)-matched allogeneic transplantation is a promising alternative strategy1, few immunological studies have been carried out with iPSCs. Here we describe an allogeneic transplantation model established using the cynomolgus monkey (Macaca fascicularis), the MHC structure of which is identical to that of humans. Fibroblast-derived iPSCs were generated from a MHC haplotype (HT4) homozygous animal and subsequently differentiated into cardiomyocytes (iPSC-CMs). Five HT4 heterozygous monkeys were subjected to myocardial infarction followed by direct intra-myocardial injection of iPSC-CMs. The grafted cardiomyocytes survived for 12 weeks with no evidence of immune rejection in monkeys treated with clinically relevant doses of methylprednisolone and tacrolimus, and showed electrical coupling with host cardiomyocytes as assessed by use of the fluorescent calcium indicator G-CaMP7.09. Additionally, transplantation of the iPSC-CMs improved cardiac contractile function at 4 and 12 weeks after transplantation; however, the incidence of ventricular tachycardia was transiently, but significantly, increased when compared to vehicle-treated controls. Collectively, our data demonstrate that allogeneic iPSC-CM transplantation is sufficient to regenerate the infarcted non-human primate heart; however, further research to control post-transplant arrhythmias is necessary.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Deleidi, M., Hargus, G., Hallett, P., Osborn, T. & Isacson, O. Development of histocompatible primate-induced pluripotent stem cells for neural transplantation. Stem Cells 29, 1052–1063 (2011)
Laflamme, M. A. & Murry, C. E. Heart regeneration. Nature 473, 326–335 (2011)
Lalit, P. A., Hei, D. J., Raval, A. N. & Kamp, T. J. Induced pluripotent stem cells for post-myocardial infarction repair: remarkable opportunities and challenges. Circ. Res. 114, 1328–1345 (2014)
Shiba, Y., Hauch, K. D. & Laflamme, M. A. Cardiac applications for human pluripotent stem cells. Curr. Pharm. Des. 15, 2791–2806 (2009)
Bach, F. H., Bach, M. L. & Sondel, P. M. Differential function of major histocompatibility complex antigens in T-lymphocyte activation. Nature 259, 273–281 (1976)
Petersdorf, E. W. The major histocompatibility complex: a model for understanding graft-versus-host disease. Blood 122, 1863–1872 (2013)
Shiina, T. et al. Discovery of novel MHC-class I alleles and haplotypes in Filipino cynomolgus macaques (Macaca fascicularis) by pyrosequencing and Sanger sequencing: Mafa-class I polymorphism. Immunogenetics 67, 563–578 (2015)
Blancher, A. et al. Study of MHC class II region polymorphism in the Filipino cynomolgus macaque population. Immunogenetics 66, 219–230 (2014)
Shiba, Y. et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322–325 (2012)
Shiba, Y. et al. Electrical integration of human embryonic stem cell-derived cardiomyocytes in a guinea pig chronic infarct model. J. Cardiovasc. Pharmacol. Ther. 19, 368–381 (2014)
Laflamme, M. A. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 25, 1015–1024 (2007)
Zhang, J. et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ. Res. 111, 1125–1136 (2012)
Tohyama, S. et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12, 127–137 (2013)
Laflamme, M. A. et al. Formation of human myocardium in the rat heart from human embryonic stem cells. Am. J. Pathol. 167, 663–671 (2005)
Minami, I. et al. A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Reports 2, 1448–1460 (2012)
Gautam, M. et al. Transplantation of adipose tissue-derived stem cells improves cardiac contractile function and electrical stability in a rat myocardial infarction model. J. Mol. Cell. Cardiol. 81, 139–149 (2015)
Li, J., Qu, J. & Nathan, R. D. Ionic basis of ryanodine’s negative chronotropic effect on pacemaker cells isolated from the sinoatrial node. Am. J. Physiol. 273, H2481–H2489 (1997)
Derks, R. A., Jankowska-Gan, E., Xu, Q. & Burlingham, W. J. Dendritic cell type determines the mechanism of bystander suppression by adaptive T regulatory cells specific for the minor antigen HA-1. J. Immunol. 179, 3443–3451 (2007)
Kwun, J. et al. Impact of leukocyte function-associated antigen-1 blockade on endogenous allospecific T cells to multiple minor histocompatibility antigen mismatched cardiac allograft. Transplantation 99, 2485–2493 (2015)
Vokaer, B. et al. Critical role of regulatory T cells in Th17-mediated minor antigen-disparate rejection. J. Immunol. 185, 3417–3425 (2010)
Kawamura, T. et al. Cardiomyocytes derived from MHC-homozygous induced pluripotent stem cells exhibit reduced allogeneic immunogenicity in MHC-matched non-human primates. Stem Cell Reports 6, 312–320 (2016)
Chong, J. J. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277 (2014)
Robinson, J., Halliwell, J. A., McWilliam, H., Lopez, R. & Marsh, S. G. IPD--the immuno polymorphism database. Nucleic Acids Res. 41, D1234–D1240 (2013)
Okita, K. et al. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells 31, 458–466 (2013)
Ohkura, M. et al. Genetically encoded green fluorescent Ca2+ indicators with improved detectability for neuronal Ca2+ signals. PLoS One 7, e51286 (2012)
Bigaud, M., Maurer, C., Vedrine, C., Puissant, B. & Blancher, A. A simple method to optimize peripheral blood mononuclear cell preparation from cynomolgus monkeys and improve mixed lymphocyte reactions. J. Pharmacol. Toxicol. Methods 50, 153–159 (2004)
Acknowledgements
We thank Y. Ichihara, N. Ishimine, Y. Karatsu, the Rigaku Corporation, Brainvision Inc. and the Keyence Corporation for assistance with the experiments and Astellas Pharma Inc. for the gift of tacrolimus. We also appreciate the scientific advice of M. A. Laflamme, J. Chong and N. Saito. This work was supported by research grants (to Y.S.) from the Japan Society for the Promotion of Science KAKENHI (grant no. 26293182), Japan Agency for Medical Research and Development, Takeda Science Foundation, Astellas Foundation for Research on Metabolic Disorders, Mochida Memorial Foundation for Medical and Pharmaceutical Research, and Japan Heart Association. The experiments to generate the G-CaMP7.09 plasmid were supported by grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) to J.N. (grant no. 26115504) and M.O. (grant no. 25116504) and a grant from the Regional Innovation Cluster Program (City Area Type, Central Saitama Area) to J.N.
Author information
Authors and Affiliations
Contributions
Y.S. designed the study. Y.S., T.G., T.Se., Y.W., H.I., Y.T., K.S. and D.I. performed all animal procedures. T.O., N.S. and Y.S. performed histological analysis. K.S. and D.I. analysed Holter ECGs. N.U., Y.K., and M.Os. performed karyotype analysis of iPSCs. T.Sh. analysed RNA sequences of cynomolgus MHC. K.O. and U.I. analysed all other data and provided administrative assistance. M.Oh. and J.N. generated the G-CaMP7.09 plasmid. In vitro fluorescent imaging studies were performed by I.M. The manuscript was written by Y.S., T.Sh., M.Oh., I.M. and N.U.
Corresponding author
Ethics declarations
Competing interests
K.S. and D.I. are employees of Ina Research, where all animal procedures in this study were performed. The remaining authors have no competing interests to declare.
Additional information
Reviewer Information Nature thanks T. Braun, K. Fukuda, T. Kamp and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Characteristics of the HT4 haplotype.
a, b, Basic structure of MHC in HT4 haplotypes. One of the cynomolgus monkeys (DrpZ5-32B-C) is strictly a ‘homozygote’ that has the A-Hp7.2 and B-Hp2 haplotypes in the Mafa-class I region and the #7 haplotype in the Mafa-class II region on both chromosomes (tentatively named ‘HT4’). c, In vitro mixed lymphoid reactions (MLR) showed that when inactivated lymphocytes from a HT4-heterozygous monkey were cocultured with active lymphocytes from a HT4-homozygous monkey, proliferation was inhibited to the level of control (only inactivated cells) or autologous (inactivated and active cells from same animal). ‘MHC mismatched’ indicates two groups of lymphocytes from two different animals with different MHC types. **P < 0.01 versus control. n = 5 per group.
Extended Data Figure 2 Generation of iPSCs from a MHC homologous cynomolgus monkey.
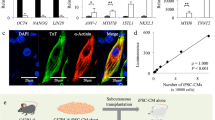
Donor iPSCs were established from skin fibroblasts by transfection of episomal vectors carrying OCT4, KLF4, SOX2 and L-MYC. a, iPSCs form typical ES-cell-like colonies. Scale bar, 50 μm. b–e, iPSCs express pluripotent markers as assessed by immunofluorescence. Scale bars, 100 μm. f, Gene expression of pluripotent markers in the iPSCs is identical to that in cynomolgus ES cells. g–i, When transplanted into immunodeficient mice, the iPSCs gave rise to teratomas manifesting all three germ layers: endoderm (intestinal epithelium), mesoderm (cartilage) and ectoderm (squamous cells). j, After expansion, the iPSCs showed normal karyotype (42, XY).
Extended Data Figure 3 Characteristics of G-CaMP7.09.
a, Schematic structure of G-CaMP7.09. Mutations are indicated with respect to G-CaMP7. RSET and M13 are tags that encode hexahistidine and a target peptide for Ca2+-bound CaM derived from myosin light chain kinase, respectively. The amino-acid numbers of EGFP and CaM are indicated in parentheses. The dynamic range of G-CaMP7.09 (Fmax/Fmin) was 19.3 ± 2.52 (n = 3) and the Kd for Ca2+ was 212 ± 6.9 nM (n = 3). b–h, In vitro fluorescence transients of G-CaMP7.09-expressing cardiomyocytes. Data are representative of three independent experiments. b, Spontaneous contraction. Scale bar, 2 s. c, The firing rate of G-CaMP signals was reduced by treatment with ryanodine, a ryanodine receptor blocker. Scale bar, 2 s. d, Addition of the L-type calcium-channel blocker, nifedipine, resulted in cessation of fluorescent transients. Scale bar, 6 s. e, Treatment with an activator of the ryanodine receptor, caffeine, induced fluorescent transients in the G-CaMP7.09-expressing iPSC-CMs. Scale bar, 6s. f, G-CaMP7.09 transients were sustained for a few minutes after spontaneous contraction and stopped by 40 mM BDM. Scale bar, 1 s. g, After cessation of spontaneous fluorescent transients, iPSC-CMs on Parafilm were stretched but no fluorescent transient was detected. Scale bar, 10 s. h, Treatment with caffeine induced G-CaMP7.09 transients again. Scale bar, 5 s.
Extended Data Figure 4 Generation and purification of cynomolgus iPS cell-derived cardiomyocytes.
a, Pilot experiments showed that cultivation of iPSC-CMs in glucose-free medium for 72 h significantly enhances cardiac purity, **P < 0.01 versus 0 h, n = 4 for each time point. Data are representative of three independent experiments. b, iPSC-CMs express the cardiac-specific marker cTnT. Scale bar, 50 μm. c, d, After multiple attempts to generate cardiomyocytes for transplantation, 2 × 109 cardiomyocytes (cTnT-positive 83.8 ± 1.0% as indicated by flow-cytometric analysis) were prepared. e, f, The cardiomyocytes were positive for GFP. g, RT–PCR analysis indicated that cTnT mRNA expression in iPSC-CMs was detectable, but lower than in the adult heart. Data are representative of three independent experiments.
Extended Data Figure 5 Study protocol and design.
a, A monolayer of cultured undifferentiated cynomolgus monkey iPSCs on a Matrigel (MG)-coated dish was treated with Matrigel. The culture medium was replaced with serum-free medium supplemented with Matrigel and activin A (AA) on day 0. On day 1 after activation, the medium was replaced with medium containing BMP4 and basic fibroblast growth factor (bFGF), and cells were cultured until day 5. On day 14, cardiomyocytes were selected by cultivation in glucose-free medium for 3 days. b, Fourteen days before transplantation, 10 female monkeys were subjected to ischaemia/reperfusion injury. Either 4 × 108 iPSC-CMs reconstituted in a prosurvival cocktail (PSC) or the PSC vehicle was injected on day 0. Cardiac μCT and UCG were performed to evaluate cardiac contractile function before and after transplantation. Additionally, BNP was measured. Spontaneous arrhythmias were monitored by Holter electrocardiogram (ECG) on days −1, 7, 14 and every other week thereafter. On day 84, all animals were euthanized, and the hearts were excised and subjected to intravital G-CaMP imaging, followed by histological analysis.
Extended Data Figure 6 Immune response following transplantation of iPS cell-derived cardiomyocytes.
a, b, iPSC-CMs were transplanted into MHC-mismatched infarcted hearts (n = 2). Animals were euthanized and the hearts were collected at 4 weeks post-transplantation. Only a small portion of grafts (GFP) showed a severe infiltration of inflammatory cells, such as CD3+ T lymphocytes. c–i, Immunohistochemical analysis of recipients of iPSC-CMs or PSC vehicle 84 days post-transplantation. The sections were stained with antibodies against CD45 (leukocytes), CD20 (B lymphocytes), CD3 (T lymphocytes) and GFP (graft). Scale bars in a–i, 200 μm.
Extended Data Figure 7 Macroscopic and microscopic analysis of iPSC-CM recipients.
a–h, All recipients of iPSC-CMs received full necropsy after euthanasia. Neither macroscopic (a–d) nor microscopic (e–h) analysis revealed any evidence of tumour formation at 12 weeks post cell transplantation. Scale bars in a–d and e–h: 10 mm and 200 μm, respectively.i–p, Additional immunohistochemical analysis of cynomolgus hearts. i, Immunohistochemistry for GFP (brown) counterstained with fast green. Scale bar, 1 mm. j, k, Picrosirius red staining of a section in close proximity to the visual field in a shows partial remuscularisation of the scar (shown in red) by grafted cardiomyocytes. l–n, Different sections (lower by 5 mm towards the apex) showing the corresponding 2 grafts from Fig. 1b. Scale bar, 200 μm. m, n, Magnified images of the grafts, scale bar, 50 μm. Note the more direct contact zone of grafted cardiomyocytes with host myocardium. o, p, Additional examples of grafted cardiomyocytes in the scar and the border zone. Scale bars, 200 μm.
Extended Data Figure 8 Summary of histological, mechanical and electrophysiological consequences.
a, Animal characteristics with histological, mechanical and calcium imaging results. b, Correlation between ejection fraction (EF) and scar area relative to left ventricular area (LV). c, Correlation between ejection fraction and graft area relative to left ventricle. d, Summary of sustained ventricular tachycardia (VT), including number of VTs, maximum duration, and maximum heart rate (HR), in the recipients of iPSC-CMs.
Extended Data Figure 9 Additional electrical analysis of hearts transplanted with iPSC-CMs.
a, b, Activation map obtained from G-CaMP7.09 transients showing the interval (in ms) between the R wave of ECG and the peak of the G-CaMP7.09 fluorescent signal. c–f, Examples of sustained and non-sustained VT in recipients of iPSC-CMs. Arrows indicate P wave during VT, suggesting atrioventricular dissociation. Scale bar, 1 s.
Extended Data Figure 10 Time course of left ventricular size and BNP levels.
a–d, Left ventricular size was analysed before transplantation (Pre-Tx), 4 weeks post-transplantation (4 w post-Tx) and 12 weeks post-transplantation (12 w post-Tx) by echocardiography (a, b) and μCT (c, d). LVEDD: left ventricular end-diastolic dimension, LVESD: left ventricular end-systolic dimension, LVEDV: left ventricular end-diastolic volume, LVESV: left ventricular end-systolic volume. n = 5 per group. #P < 0.05; ##P < 0.01. * P < 0.05; **P < 0.01 versus Pre-TX. e, BNP was measured on days 0 (14 days after myocardial infarction), 28, 56 and 84. No significant difference was detected between recipients of iPSC-CMs and recipients of PSC vehicle at any time point. *P < 0.05 versus day 0.
Supplementary information
Supplementary Information
This file contains gel source data for Extended Data Figures 2 and 4. (PDF 1317 kb)
In vitro fluorescent imaging of G-CaMP7.09-expressing cynomolgus iPSC-CMs
Monolayer-cultured cardiomyocytes exhibit robust fluorescent flashes in synchrony with their contraction. (MP4 10625 kb)
In vitro experiments with G-CaMP7.09-expressing iPSC-CMs by using nifedipine, ryanodine, and caffeine
Representative video of G-CaMP7.09 fluorescent transients and contraction of cardiomyocytes before and after treatment with the L-type calcium-channel blocker nifedipine, the ryanodine receptor blocker ryanodine, and the ryanodine receptor activator caffeine. (MP4 15870 kb)
In vitro G-CaMP7.09 transients sustained after cessation of spontaneous contraction of iPSC-CMs by BDM.
Treatment with 40 mM BDM resulted in cessation of spontaneous contraction of iPSC-CMs, but G-CaMP7.09 fluorescent transients were sustained for a few minutes. (MP4 6161 kb)
Myocardial ischemia/reperfusion model in cynomolgus monkey
The myocardial infarction model was produced by 3 h of ischemia followed by reperfusion using polyethylene tubing 2 weeks before transplantation. (MP4 20319 kb)
Intravital imaging of G-CaMP7.09-expressing iPSC-CMs in cynomolgus heart
G-CaMP7.09+ iPSC-CMs were transplanted into infarcted cynomolgus hearts. The hearts were excised and mechanically arrested ex vivo by perfusion with BDM on a Langendorff apparatus at 12 weeks post-transplantation. All graft regions exhibited cyclic changes in fluorescent intensity that occurred synchronously in a 1:1 relationship with the host ECG when the heart beat spontaneously or was electrically paced at rates from 3 to 5 Hz. Note that some, but not all hearts could be paced up to 5 Hz. (MP4 9721 kb)
Cardiac contractile function assessed by mCT at 4 weeks post-transplantation
Cardiac function was assessed by mCT pre- and post-transplantation. The first and second segments of the video show left ventricular contractions of the short axis at the base and apex, respectively. Note that while contraction at the base is similar, that at the apex in iPSC-CM recipients looks better than that in PSC-vehicle recipients. The last segment represents a long-axis view of the left ventricle. (MP4 3427 kb)
Rights and permissions
About this article
Cite this article
Shiba, Y., Gomibuchi, T., Seto, T. et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538, 388–391 (2016). https://doi.org/10.1038/nature19815
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature19815
This article is cited by
-
Pre-clinical evaluation of the efficacy and safety of human induced pluripotent stem cell-derived cardiomyocyte patch
Stem Cell Research & Therapy (2024)
-
The role of cardiac pericytes in health and disease: therapeutic targets for myocardial infarction
Nature Reviews Cardiology (2024)
-
JAK2 as a surface marker for enrichment of human pluripotent stem cells-derived ventricular cardiomyocytes
Stem Cell Research & Therapy (2023)
-
Exosomes secreted by endothelial cells derived from human induced pluripotent stem cells improve recovery from myocardial infarction in mice
Stem Cell Research & Therapy (2023)
-
Therapeutic angiogenesis and tissue revascularization in ischemic vascular disease
Journal of Biological Engineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.